In vitro and in vivo evaluation of small interference RNA-mediated gynaecophoral canal protein silencing in Schistosoma japonicum
- PMID: 19288459
- PMCID: PMC7166781
- DOI: 10.1002/jgm.1314
In vitro and in vivo evaluation of small interference RNA-mediated gynaecophoral canal protein silencing in Schistosoma japonicum
Abstract
Background: Schistosomiasis causes liver and intestinal damage and can be very debilitating. The pairing of a male worm with a female worm residing in the gynaecophoral canal of male plays a critical role in the development of female parasite. Because the male specific gynaecophoral canal protein of Schistosoma japonicum (SjGCP) is found in significant quantities in the adult female worm after pairing, it could play an important role in parasite pairing.
Methods: In the present study, three small interfering (si)RNA duplexes targeting the SjGCP gene were designed, synthesized and the silencing effects were evaluated in vitro as well as in mice infected with S. japonicum in vivo.
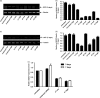
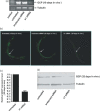
Results: In vitro studies using semi-quantitative reverse transcriptase-polymerase chain reaction (RT-PCR) and real-time RT-PCR revealed the reduction of SjGCP at the transcript level. Similarly, western blotting and immunofluorescence studies showed its reduction at the protein level after treatment of parasites with siRNAs. At a concentration of 200 nM, two siRNAs totally abolished the parasite pairing. To evaluate such a pairing inhibitory effect in vivo, mice infected with S. japonicum were treated with siRNA and both parasite pairing and burden were evaluated. In vivo tests confirmed the in vitro silencing effect of SjGCP siRNA and revealed that the systemic delivery of siRNA significantly inhibited early parasite pairing and the associated burden.
Conclusions: Our preliminary results demonstrated that the SjGCP plays an important role in pairing and subsequent development in S. japonicum, and its silencing might have potential as a therapeutic approach for controlling schistosomiasis.
(c) 2009 John Wiley & Sons, Ltd.
Figures


References
-
- Smithers SR, Terry RJ. The immunology of schistosomiasis. Adv Parasitol 1969; 7: 41–93. - PubMed
-
- Basch PF. Schistosoma mansoni: nucleic acid synthesis in immature females from single‐sex infections, paired in vitro with intact males and male segments. Comp Biochem Physiol B 1988; 90: 389–392. - PubMed
-
- Cheng GF, Lin JJ, Feng XG, et al. Proteomic analysis of differentially expressed proteins between the male and female worm of Schistosoma japonicum after pairing. Proteomics 2005; 5: 511–521. - PubMed
-
- Den Hollander JE, Erasmus DA. Schistosoma mansoni: male stimulation and DNA synthesis by the female. Parasitology 1985; 91: 449–457. - PubMed
-
- Gupta BC, Basch PF. The role of Schistosoma mansoni males in feeding and development of female worms. J Parasitol 1987; 73: 481–486. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

