Efficacy of systemic morpholino exon-skipping in Duchenne dystrophy dogs
- PMID: 19288467
- PMCID: PMC5951302
- DOI: 10.1002/ana.21627
Efficacy of systemic morpholino exon-skipping in Duchenne dystrophy dogs
Abstract
Objective: Duchenne muscular dystrophy (DMD) is caused by the inability to produce dystrophin protein at the myofiber membrane. A method to rescue dystrophin production by antisense oligonucleotides, termed exon-skipping, has been reported for the mdx mouse and in four DMD patients by local intramuscular injection. We sought to test efficacy and toxicity of intravenous oligonucleotide (morpholino)-induced exon skipping in the DMD dog model.
Methods: We tested a series of antisense drugs singly and as cocktails, both in primary cell culture, and two in vivo delivery methods (intramuscular injection and systemic intravenous injection). The efficiency and efficacy of multiexon skipping (exons 6-9) were tested at the messenger RNA, protein, histological, and clinical levels.
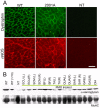
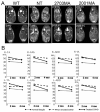
Results: Weekly or biweekly systemic intravenous injections with a three-morpholino cocktail over the course of 5 to 22 weeks induced therapeutic levels of dystrophin expression throughout the body, with an average of about 26% normal levels. This was accompanied by reduced inflammatory signals examined by magnetic resonance imaging and histology, improved or stabilized timed running tests, and clinical symptoms. Blood tests indicated no evidence of toxicity.
Interpretation: This is the first report of widespread rescue of dystrophin expression to therapeutic levels in the dog model of DMD. This study also provides a proof of concept for systemic multiexon-skipping therapy. Use of cocktails of morpholino, as shown here, allows broader application of this approach to a greater proportion of DMD patients (90%) and also offers the prospect of selecting deletions that optimize the functionality of the dystrophin protein.
Figures





References
-
- Hoffman EP, Brown RH, Jr., Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. - PubMed
-
- Matsumura K, Campbell KP. Dystrophin-glycoprotein complex: its role in the molecular pathogenesis of muscular dystrophies. Muscle Nerve. 1994;17:2–15. - PubMed
-
- England SB, Nicholson LV, Johnson MA, et al. Very mild muscular dystrophy associated with the deletion of 46% of dystrophin. Nature. 1990;343:180–182. - PubMed
-
- Dunckley MGMM, Villiet P, Eperon IC, Dickson G. Modification of splicing in the dystrophin gene in cultured Mdx muscle cells by antisense oligoribonucleotides. Hum Mol Genet. 1998;7:1083–1090. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

