Profile of native N-linked glycan structures from human serum using high performance liquid chromatography on a microfluidic chip and time-of-flight mass spectrometry
- PMID: 19288519
- PMCID: PMC2765869
- DOI: 10.1002/pmic.200800249
Profile of native N-linked glycan structures from human serum using high performance liquid chromatography on a microfluidic chip and time-of-flight mass spectrometry
Abstract
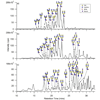
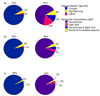
Protein glycosylation involves the addition of monosaccharides in a stepwise process requiring no glycan template. Therefore, identifying the numerous glycoforms, including isomers, can help elucidate the biological function(s) of particular glycans. A method to assess the diversity of the N-linked oligosaccharides released from human serum without derivatization has been developed using on-line nanoLC and high resolution TOF MS. The N-linked oligosaccharides were analyzed with MALDI FT-ICR MS and microchip LC MS (HPLC-Chip/TOF MS). Two microfluidic chips were employed, the glycan chip (40 nL enrichment column, 43 x 0.075 mm(2) i.d. analytical column) and the high capacity chip (160 nL enrichment column, 140 x 0.075 mm(2) i.d. analytical column), both with graphitized carbon as the stationary phase. Both chips offered good sensitivity and reproducibility in separating a heterogeneous mixture of neutral and anionic oligosaccharides between injections. Increasing the length and volume of the enrichment and the analytical columns improved resolution of the peaks. Complex type N-linked oligosaccharides were the most abundant oligosaccharides in human serum accounting for approximately 96% of the total glycans identified, while hybrid and high mannose type oligosaccharides comprise the remaining approximately 4%.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Dwek RA. Glycobiology: Toward Understanding the Function of Sugars. Chem Rev. 1996;96:683–720. - PubMed
-
- Varki A, Cummings R, Esko J, Freeze H, et al. Essentials of Glycobiology. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 1999. - PubMed
-
- Ratner DM, Adams EW, Disney MD, Seeberger PH. Tools for glycomics: mapping interactions of carbohydrates in biological systems. Chembiochem. 2004;5:1375–1383. - PubMed
-
- Zhang XL. Roles of glycans and glycopeptides in immune system and immune-related diseases. Curr Med Chem. 2006;13:1141–1147. - PubMed
-
- Gagneux P, Varki A. Evolutionary considerations in relating oligosaccharide diversity to biological function. Glycobiology. 1999;9:747–755. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

