The effects of ADF/cofilin and profilin on the conformation of the ATP-binding cleft of monomeric actin
- PMID: 19289059
- PMCID: PMC2865994
- DOI: 10.1016/j.bpj.2008.12.3906
The effects of ADF/cofilin and profilin on the conformation of the ATP-binding cleft of monomeric actin
Abstract
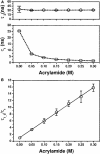
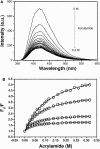
Actin depolymerizing factor (ADF)/cofilin and profilin are small actin-binding proteins, which have central roles in cytoskeletal dynamics in all eukaryotes. When bound to an actin monomer, ADF/cofilins inhibit the nucleotide exchange, whereas most profilins accelerate the nucleotide exchange on actin monomers. In this study the effects of ADF/cofilin and profilin on the accessibility of the actin monomer's ATP-binding pocket was investigated by a fluorescence spectroscopic method. The fluorescence of the actin bound epsilon-ATP was quenched with a neutral quencher (acrylamide) in steady-state and time dependent experiments, and the data were analyzed with a complex form of the Stern-Volmer equation. The experiments revealed that in the presence of ADF/cofilin the accessibility of the bound epsilon-ATP decreased, indicating a closed and more compact ATP-binding pocket induced by the binding of ADF/cofilin. In the presence of profilin the accessibility of the bound epsilon-ATP increased, indicating a more open and approachable protein matrix around the ATP-binding pocket. The results of the fluorescence quenching experiments support a structural mechanism regarding the regulation of the nucleotide exchange on actin monomers by ADF/cofilin and profilin.
Figures



Similar articles
-
The effect of ADF/cofilin and profilin on the dynamics of monomeric actin.Biochim Biophys Acta. 2013 Oct;1834(10):2010-9. doi: 10.1016/j.bbapap.2013.06.006. Epub 2013 Jul 8. Biochim Biophys Acta. 2013. PMID: 23845993
-
Synergy between actin depolymerizing factor/cofilin and profilin in increasing actin filament turnover.J Biol Chem. 1998 Oct 2;273(40):25602-11. doi: 10.1074/jbc.273.40.25602. J Biol Chem. 1998. PMID: 9748225
-
Identification of Arabidopsis cyclase-associated protein 1 as the first nucleotide exchange factor for plant actin.Mol Biol Cell. 2007 Aug;18(8):3002-14. doi: 10.1091/mbc.e06-11-1041. Epub 2007 May 30. Mol Biol Cell. 2007. PMID: 17538023 Free PMC article.
-
Tropomyosin and ADF/cofilin as collaborators and competitors.Adv Exp Med Biol. 2008;644:232-49. doi: 10.1007/978-0-387-85766-4_18. Adv Exp Med Biol. 2008. PMID: 19209826 Review.
-
The ADF/cofilin family: actin-remodeling proteins.Genome Biol. 2002;3(5):reviews3007. doi: 10.1186/gb-2002-3-5-reviews3007. Epub 2002 Apr 26. Genome Biol. 2002. PMID: 12049672 Free PMC article. Review.
Cited by
-
A nucleotide state-sensing region on actin.J Biol Chem. 2010 Aug 13;285(33):25591-601. doi: 10.1074/jbc.M110.123869. Epub 2010 Jun 8. J Biol Chem. 2010. PMID: 20530485 Free PMC article.
-
Cdk12 maintains the integrity of adult axons by suppressing actin remodeling.Cell Death Discov. 2023 Sep 20;9(1):348. doi: 10.1038/s41420-023-01642-4. Cell Death Discov. 2023. PMID: 37730761 Free PMC article.
-
Profilin is associated with transcriptionally active genes.Nucleus. 2012 May-Jun;3(3):290-9. doi: 10.4161/nucl.20327. Epub 2012 May 1. Nucleus. 2012. PMID: 22572953 Free PMC article.
-
Actin Filament Barbed-End Depolymerization by Combined Action of Profilin, Cofilin, and Twinfilin.PRX Life. 2024 Jul-Sep;2(3):033002. doi: 10.1103/prxlife.2.033002. Epub 2024 Jul 16. PRX Life. 2024. PMID: 40777877 Free PMC article.
-
Crystal structures explain functional differences in the two actin depolymerization factors of the malaria parasite.J Biol Chem. 2011 Aug 12;286(32):28256-64. doi: 10.1074/jbc.M111.211730. J Biol Chem. 2011. PMID: 21832095 Free PMC article.
References
-
- dos Remedios C.G., Chhabra D., Kekic M., Dedova I.V., Tsubakihara M. Actin binding proteins: regulation of cytoskeletal microfilaments. Physiol. Rev. 2003;83:433–473. - PubMed
-
- Paavilainen V.O., Bertling E., Falck S., Lappalainen P. Regulation of cytoskeletal dynamics by actin-monomer-binding proteins. Trends Cell Biol. 2004;14:386–394. - PubMed
-
- Nishida E., Maekawa S., Sakai H. Cofilin, a protein in porcine brain that binds to actin filaments and inhibits their interactions with myosin and tropomyosin. Biochemistry. 1984;23:5307–5313. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

