The effects of ADF/cofilin and profilin on the conformation of the ATP-binding cleft of monomeric actin
- PMID: 19289059
- PMCID: PMC2865994
- DOI: 10.1016/j.bpj.2008.12.3906
The effects of ADF/cofilin and profilin on the conformation of the ATP-binding cleft of monomeric actin
Abstract
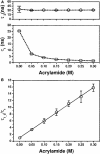
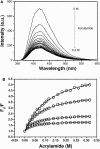
Actin depolymerizing factor (ADF)/cofilin and profilin are small actin-binding proteins, which have central roles in cytoskeletal dynamics in all eukaryotes. When bound to an actin monomer, ADF/cofilins inhibit the nucleotide exchange, whereas most profilins accelerate the nucleotide exchange on actin monomers. In this study the effects of ADF/cofilin and profilin on the accessibility of the actin monomer's ATP-binding pocket was investigated by a fluorescence spectroscopic method. The fluorescence of the actin bound epsilon-ATP was quenched with a neutral quencher (acrylamide) in steady-state and time dependent experiments, and the data were analyzed with a complex form of the Stern-Volmer equation. The experiments revealed that in the presence of ADF/cofilin the accessibility of the bound epsilon-ATP decreased, indicating a closed and more compact ATP-binding pocket induced by the binding of ADF/cofilin. In the presence of profilin the accessibility of the bound epsilon-ATP increased, indicating a more open and approachable protein matrix around the ATP-binding pocket. The results of the fluorescence quenching experiments support a structural mechanism regarding the regulation of the nucleotide exchange on actin monomers by ADF/cofilin and profilin.
Figures



References
-
- dos Remedios C.G., Chhabra D., Kekic M., Dedova I.V., Tsubakihara M. Actin binding proteins: regulation of cytoskeletal microfilaments. Physiol. Rev. 2003;83:433–473. - PubMed
-
- Paavilainen V.O., Bertling E., Falck S., Lappalainen P. Regulation of cytoskeletal dynamics by actin-monomer-binding proteins. Trends Cell Biol. 2004;14:386–394. - PubMed
-
- Nishida E., Maekawa S., Sakai H. Cofilin, a protein in porcine brain that binds to actin filaments and inhibits their interactions with myosin and tropomyosin. Biochemistry. 1984;23:5307–5313. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

