Concentration gradient effects of sodium and lithium ions and deuterium isotope effects on the activities of H+-ATP synthase from chloroplasts
- PMID: 19289072
- PMCID: PMC2907681
- DOI: 10.1016/j.bpj.2008.12.3910
Concentration gradient effects of sodium and lithium ions and deuterium isotope effects on the activities of H+-ATP synthase from chloroplasts
Abstract
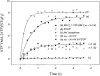
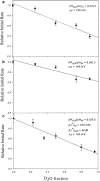
We explored the concentration gradient effects of the sodium and lithium ions and the deuterium isotope's effects on the activities of H(+)-ATP synthase from chloroplasts (CF(0)F(1)). We found that the sodium concentration gradient can drive the ATP synthesis reaction of CF(0)F(1). In contrast, the lithium ion can be an efficient enzyme-inhibitor by blocking the entrance channel of the ion translocation pathway in CF(0). In the presence of sodium or lithium ions and with the application of a membrane potential, unexpected enzyme behaviors of CF(0)F(1) were evident. To account for these observations, we propose that both of the sodium and lithium ions could undergo localized hydrolysis reactions in the chemical environment of the ion channel of CF(0). The protons generated locally could proceed to complete the ion translocation process in the ATP synthesis reaction of CF(0)F(1). Experimental and theoretical deuterium isotope effects of the localized hydrolysis on the activities of CF(0)F(1), and the energetics of these related reactions, support this proposed mechanism. Our experimental observations could be understood in the framework of the well-established ion translocation models for the H(+)-ATP synthase from Escherichia coli, and the Na(+)-ATP synthase from Propionigenium modestum and Ilyobacter tartaricus.
Figures





References
-
- Mitchell P. Coupling of phosphorylation to electron and hydrogen transfer by chemi-osmotic type of mechanism. Nature. 1961;191:144–148. - PubMed
-
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol. Rev. Camb. Philos. Soc. 1966;41:445–502. - PubMed
-
- Possmayer F.E., Gräber P. The pHin and pHout dependence of the rate of ATP synthesis catalyzed by the chloroplast H+-ATPase, CF0F1, in proteoliposomes. J. Biol. Chem. 1994;269:1896–1904. - PubMed
-
- Fischer S., Etzold C., Turina P., Deckers-Hebestreit G., Altendorf K. ATP synthesis catalyzed by the ATP synthase of Escherichia coli reconstituted into liposomes. Eur. J. Biochem. 1994;225:167–172. - PubMed
-
- Fischer S., Gräber P. Comparison of ΔpH- and Δϕ-driven ATP synthesis catalyzed by the H(+)-ATPases from Escherichia coli or chloroplasts reconstituted into liposomes. FEBS Lett. 1999;457:327–332. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

