Regulation of integrin activity and signalling
- PMID: 19289150
- PMCID: PMC2734279
- DOI: 10.1016/j.bbagen.2009.03.007
Regulation of integrin activity and signalling
Abstract
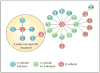
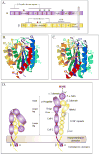
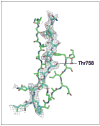
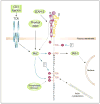
The ability of cells to attach to each other and to the extracellular matrix is of pivotal significance for the formation of functional organs and for the distribution of cells in the body. Several molecular families of proteins are involved in adhesion, and recent work has substantially improved our understanding of their structures and functions. Also, these molecules are now being targeted in the fight against disease. However, less is known about how their activity is regulated. It is apparent that among the different classes of adhesion molecules, the integrin family of adhesion receptors is unique in the sense that they constitute a large group of widely distributed receptors, they are unusually complex and most importantly their activities are strictly regulated from the inside of the cell. The activity regulation is achieved by a complex interplay of cytoskeletal proteins, protein kinases, phosphatases, small G proteins and adaptor proteins. Obviously, we are only in the beginning of our understanding of how the integrins function, but already now fascinating details have become apparent. Here, we describe recent progress in the field, concentrating mainly on mechanistical and structural studies of integrin regulation. Due to the large number of articles dealing with integrins, we focus on what we think are the most exciting and rewarding directions of contemporary research on cell adhesion and integrins.
Figures




References
-
- Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991;251:1451–1455. - PubMed
-
- González-Amaro R, Sanchez-Madrid F. Cell adhesion molecules: selectins and integrins. Crit Rev Immunol. 1999;19:389–429. - PubMed
-
- Yona S, Lin HH, Siu WO, Gordon S, Stacey M. Adhesion-GPCRs: emerging roles for novel receptors. Trends Biochem Sci. 2008;33:491–500. - PubMed
-
- Cunningham BA, Hemperly JJ, Murray BA, Prediger EA, Brackenbury R, Edelman GM. Neural cell adhesion molecule: structure, immunoglobulin-like domains, cell surface modulation, and alternative RNA splicing. Science. 1987;236:799–806. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

