Major depression and antidepressant treatment: impact on pregnancy and neonatal outcomes
- PMID: 19289451
- PMCID: PMC4426499
- DOI: 10.1176/appi.ajp.2008.08081170
Major depression and antidepressant treatment: impact on pregnancy and neonatal outcomes
Abstract
Objective: Selective serotonin reuptake inhibitor (SSRI) use during pregnancy incurs a low absolute risk for major malformations; however, other adverse outcomes have been reported. Major depression also affects reproductive outcomes. This study examined whether 1) minor physical anomalies, 2) maternal weight gain and infant birth weight, 3) preterm birth, and 4) neonatal adaptation are affected by SSRI or depression exposure.
Method: This prospective observational investigation included maternal assessments at 20, 30, and 36 weeks of gestation. Neonatal outcomes were obtained by blinded review of delivery records and infant examinations. Pregnant women (N=238) were categorized into three mutually exclusive exposure groups: 1) no SSRI, no depression (N=131); 2) SSRI exposure (N=71), either continuous (N=48) or partial (N=23); and 3) major depressive disorder (N=36), either continuous (N=14) or partial (N=22). The mean depressive symptom level of the group with continuous depression and no SSRI exposure was significantly greater than for all other groups, demonstrating the expected treatment effect of SSRIs. Main outcomes were minor physical anomalies, maternal weight gain, infant birth weight, pregnancy duration, and neonatal characteristics.
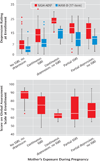
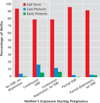
Results: Infants exposed to either SSRIs or depression continuously across gestation were more likely to be born preterm than infants with partial or no exposure. Neither SSRI nor depression exposure increased risk for minor physical anomalies or reduced maternal weight gain. Mean infant birth weights were equivalent. Other neonatal outcomes were similar, except 5-minute Apgar scores.
Conclusions: For depressed pregnant women, both continuous SSRI exposure and continuous untreated depression were associated with preterm birth rates exceeding 20%.
Trial registration: ClinicalTrials.gov NCT00279370.
Figures

Comment in
-
Assessing risk and benefit: to treat or not to treat major depression during pregnancy with antidepressant medication.Am J Psychiatry. 2009 May;166(5):512-4. doi: 10.1176/appi.ajp.2009.09020251. Am J Psychiatry. 2009. PMID: 19411375 No abstract available.
-
Antidepressant use and preterm birth.Am J Psychiatry. 2009 Oct;166(10):1189; author reply 1189-90. doi: 10.1176/appi.ajp.2009.09050712. Am J Psychiatry. 2009. PMID: 19797450 No abstract available.
-
To treat or not to treat perinatal depression with antidepressant medication: effects on infant growth.Am J Psychiatry. 2013 May;170(5):453-4. doi: 10.1176/appi.ajp.2013.13010118. Am J Psychiatry. 2013. PMID: 23511843 No abstract available.
References
-
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS National Comorbidity Survey Replication. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–3105. - PubMed
-
- Reefhuis J, Rasmussen SA, Friedman JM. Selective serotonin-reuptake inhibitors and persistent pulmonary hypertension of the newborn; reply of Chambers C, Hernandez-Diaz S, Mitchell AA (letter) N Engl J Med. 2006;354:2188–2190. - PubMed
-
- Kulin NA, Pastuszak A, Sage SR, Schick-Boschetto B, Spivey G, Feldkamp M, Ormond K, Matsui D, Stein-Schechman AK, Cook L, Brochu J, Rieder M, Koren G. Pregnancy outcome following maternal use of the new selective serotonin reuptake inhibitors: a prospective controlled multicenter study. JAMA. 1998;279:609–610. - PubMed
-
- Nulman I, Rovet J, Stewart DE, Wolpin J, Gardner HA, Theis JG, Kulin N, Koren G. Neurodevelopment of children exposed in utero to antidepressant drugs. N Engl J Med. 1997;336:258–262. - PubMed
-
- Pastuszak A, Schick-Boschetto B, Zuber C, Feldkamp M, Pinelli M, Sihn S, Donnenfeld A, McCormack M, Leen-Mitchell M, Woodland C, et al. Pregnancy outcome following first-trimester exposure to fluoxetine (Prozac) JAMA. 1993;269:2246–2248. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- R25 MH060473/MH/NIMH NIH HHS/United States
- K12 HD043441/HD/NICHD NIH HHS/United States
- K12 HD-043441/HD/NICHD NIH HHS/United States
- K23 MH064561/MH/NIMH NIH HHS/United States
- R01 MH-60335/MH/NIMH NIH HHS/United States
- R01 MH075921/MH/NIMH NIH HHS/United States
- R25 MH-060473/MH/NIMH NIH HHS/United States
- K23 MH-64561/MH/NIMH NIH HHS/United States
- K01 MH-074092/MH/NIMH NIH HHS/United States
- R01 MH079164/MH/NIMH NIH HHS/United States
- R01 MH071825/MH/NIMH NIH HHS/United States
- R01 MH-075921/MH/NIMH NIH HHS/United States
- K01 MH074092/MH/NIMH NIH HHS/United States
- R01 MH060335/MH/NIMH NIH HHS/United States
- R01 MH-071825/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

