Inhibition of Th17 cells regulates autoimmune diabetes in NOD mice
- PMID: 19289457
- PMCID: PMC2682686
- DOI: 10.2337/db08-1113
Inhibition of Th17 cells regulates autoimmune diabetes in NOD mice
Abstract
Objective: The T helper 17 (Th17) population, a subset of CD4-positive T-cells that secrete interleukin (IL)-17, has been implicated in autoimmune diseases, including multiple sclerosis and lupus. Therapeutic agents that target the Th17 effector molecule IL-17 or directly inhibit the Th17 population (IL-25) have shown promise in animal models of autoimmunity. The role of Th17 cells in type 1 diabetes has been less clear. The effect of neutralizing anti-IL-17 and recombinant IL-25 on the development of diabetes in NOD mice, a model of spontaneous autoimmune diabetes, was investigated in this study.
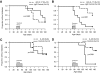
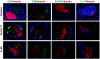
Research design and methods and results: Although treatment with either anti-IL-17 or IL-25 had no effect on diabetes development in young (<5 weeks) NOD mice, either intervention prevented diabetes when treatment was started at 10 weeks of age (P < 0.001). Insulitis scoring and immunofluorescence staining revealed that both anti-IL-17 and IL-25 significantly reduced peri-islet T-cell infiltrates. Both treatments also decreased GAD65 autoantibody levels. Analysis of pancreatic lymph nodes revealed that both treatments increased the frequency of regulatory T-cells. Further investigation demonstrated that IL-25 therapy was superior to anti-IL-17 during mature diabetes because it promoted a period of remission from new-onset diabetes in 90% of treated animals. Similarly, IL-25 delayed recurrent autoimmunity after syngeneic islet transplantation, whereas anti-IL-17 was of no benefit. GAD65-specific ELISpot and CD4-positive adoptive transfer studies showed that IL-25 treatment resulted in a T-cell-mediated dominant protective effect against autoimmunity.
Conclusions: These studies suggest that Th17 cells are involved in the pathogenesis of autoimmune diabetes. Further development of Th17-targeted therapeutic agents may be of benefit in this disease.
Figures

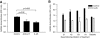


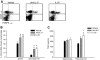
References
-
- Mouzaki A, Deraos S, Chatzantoni K: Advances in the treatment of autoimmune diseases; cellular activity, type-1/type-2 cytokine secretion patterns and their modulation by therapeutic peptides. Curr Med Chem 2005; 12: 1537– 1550 - PubMed
-
- van Roon JA, Bijlsma JW, Lafeber FP: Suppression of inflammation and joint destruction in rheumatoid arthritis may require a concerted action of Th2 cytokines. Curr Opin Investig Drugs 2002; 3: 1011– 1016 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

