Protein kinase D mediates mitogenic signaling by Gq-coupled receptors through protein kinase C-independent regulation of activation loop Ser744 and Ser748 phosphorylation
- PMID: 19289471
- PMCID: PMC2679443
- DOI: 10.1074/jbc.M806554200
Protein kinase D mediates mitogenic signaling by Gq-coupled receptors through protein kinase C-independent regulation of activation loop Ser744 and Ser748 phosphorylation
Abstract
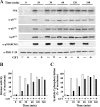
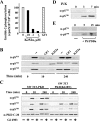
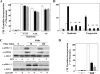
Rapid protein kinase D (PKD) activation and phosphorylation via protein kinase C (PKC) have been extensively documented in many cell types cells stimulated by multiple stimuli. In contrast, little is known about the role and mechanism(s) of a recently identified sustained phase of PKD activation in response to G protein-coupled receptor agonists. To elucidate the role of biphasic PKD activation, we used Swiss 3T3 cells because PKD expression in these cells potently enhanced duration of ERK activation and DNA synthesis in response to G(q)-coupled receptor agonists. Cell treatment with the preferential PKC inhibitors GF109203X or Gö6983 profoundly inhibited PKD activation induced by bombesin stimulation for <15 min but did not prevent PKD catalytic activation induced by bombesin stimulation for longer times (>60 min). The existence of sequential PKC-dependent and PKC-independent PKD activation was demonstrated in 3T3 cells stimulated with various concentrations of bombesin (0.3-10 nm) or with vasopressin, a different G(q)-coupled receptor agonist. To gain insight into the mechanisms involved, we determined the phosphorylation state of the activation loop residues Ser(744) and Ser(748). Transphosphorylation targeted Ser(744), whereas autophosphorylation was the predominant mechanism for Ser(748) in cells stimulated with G(q)-coupled receptor agonists. We next determined which phase of PKD activation is responsible for promoting enhanced ERK activation and DNA synthesis in response to G(q)-coupled receptor agonists. We show, for the first time, that the PKC-independent phase of PKD activation mediates prolonged ERK signaling and progression to DNA synthesis in response to bombesin or vasopressin through a pathway that requires epidermal growth factor receptor-tyrosine kinase activity. Thus, our results identify a novel mechanism of G(q)-coupled receptor-induced mitogenesis mediated by sustained PKD activation through a PKC-independent pathway.
Figures




References
-
- Rozengurt, E., Rey, O., and Waldron, R. T. (2005) J. Biol. Chem. 280 13205–13208 - PubMed
-
- Iglesias, T., and Rozengurt, E. (1998) J. Biol. Chem. 273 410–416 - PubMed
-
- Iglesias, T., and Rozengurt, E. (1999) FEBS Lett. 454 53–56 - PubMed
-
- Waldron, R. T., and Rozengurt, E. (2003) J. Biol. Chem. 278 154–163 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

