REV3L confers chemoresistance to cisplatin in human gliomas: the potential of its RNAi for synergistic therapy
- PMID: 19289490
- PMCID: PMC2802399
- DOI: 10.1215/15228517-2009-015
REV3L confers chemoresistance to cisplatin in human gliomas: the potential of its RNAi for synergistic therapy
Abstract
The REV3L gene, encoding the catalytic subunit of human polymerase zeta, plays a significant role in the cytotoxicity, mutagenicity, and chemoresistance of certain tumors. However, the role of REV3L in regulating the sensitivity of glioma cells to chemotherapy remains unknown. In this study, we investigated the expression of the REV3L gene in 10 normal brain specimens and 30 human glioma specimens and examined the value of REV3L as a potential modulator of cellular response to various DNA-damaging agents. Reverse transcriptase PCR/real-time PCR analysis revealed that REV3L was overexpressed in human gliomas compared with normal brain tissues. A glioma cell model with stable overexpression of REV3L was used to probe the role of REV3L in cisplatin treatment; upregulation of REV3L markedly attenuated cisplatin-induced apoptosis of the mitochondrial apoptotic pathway. We therefore assessed the REV3L-targeted treatment modality that combines suppression of REV3L expression using RNA interference (RNAi) with the cytotoxic effects of DNA-damaging agents. Downregulation of REV3L expression significantly enhanced the sensitivity of glioma cells to cisplatin, as evidenced by the increased apoptosis rate and marked alterations in the anti-apoptotic proteins B-cell lymphoma 2 (Bcl-2) and B-cell lymphoma-extra large (Bcl-xl) and proapoptotic Bcl-2-associated x protein (Bax) expression levels, and reduced mutation frequencies in surviving glioma cells. These results suggest that REV3L may potentially contribute to gliomagenesis and play a crucial role in regulating cellular response to the DNA cross-linking agent cisplatin. Our findings indicate that RNAi targeting REV3L combined with chemotherapy has synergistic therapeutic effects on glioma cells, which warrants further investigation as an effective novel therapeutic regimen for patients with this malignancy.
Figures




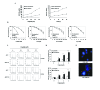

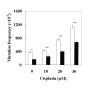
References
-
- Behin A, Hoang-Xuan K, Carpentier AF, Delattre JY. Primary brain tumours in adults. Lancet. 2003;361:323–331. - PubMed
-
- Furnari FB, Fenton T, Bachoo RM, et al. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21:2683–2710. - PubMed
-
- Barzon L, Zanusso M, Colombo F, Palu G. Clinical trials of gene therapy, virotherapy, and immunotherapy for malignant gliomas. Cancer Gene Ther. 2006;13:539–554. - PubMed
-
- DeAngelis LM. Brain tumors. N Engl J Med. 2001;344:114–123. - PubMed
-
- Okada H, Pollack IF. Cytokine gene therapy for malignant glioma. Expert Opin Biol Ther. 2004;4:1609–1620. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

