Designing therapies against experimental visceral leishmaniasis by modulating the membrane fluidity of antigen-presenting cells
- PMID: 19289510
- PMCID: PMC2687339
- DOI: 10.1128/IAI.00057-09
Designing therapies against experimental visceral leishmaniasis by modulating the membrane fluidity of antigen-presenting cells
Abstract
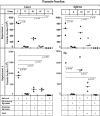
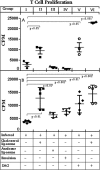
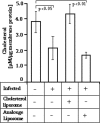
The membrane fluidity of antigen-presenting cells (APCs) has a significant bearing on T-cell-stimulating ability and is dependent on the cholesterol content of the membrane. The relationship, if any, between membrane fluidity and defective cell-mediated immunity in visceral leishmaniasis has been investigated. Systemic administration of cholesterol by liposome delivery (cholesterol liposomes) in Leishmania donovani-infected hamsters was found to cure the infection. Splenic macrophages as a prototype of APCs in infected hamsters had decreased membrane cholesterol and an inability to drive T cells, which was corrected by cholesterol liposome treatment. The effect was cholesterol specific because liposomes made up of the analogue 4-cholesten-3-one provided almost no protection. Infection led to increases in interleukin-10 (IL-10), transforming growth factor beta, and IL-4 signals and concomitant decreases in gamma interferon (IFN-gamma), tumor necrosis factor alpha, and inducible NO synthase signals, which reverted upon cholesterol liposome treatment. The antileishmanial T-cell repertoire, whose expansion appeared to be associated with protection, was presumably type Th1, as shown by enhanced IFN-gamma signals and the predominance of the immunoglobulin G2 isotype. The protected group produced significantly more reactive oxygen species and NO than the infected groups, which culminated in killing of L. donovani parasites. Therefore, cholesterol liposome treatment may be yet another simple strategy to enhance the cell-mediated immune response to L. donovani infection. To our knowledge, this is the first report on the therapeutic effect of cholesterol liposomes in any form of the disease.
Figures





References
-
- Amer, A. O., and M. S. Swanson. 2002. Phagosome of one's own: a microbial guide to life in the macrophage. Curr. Opin. Microbiol. 556-61. - PubMed
-
- Amundson, D. M., and M. Zhou. 1999. Fluorometric method for the enzymatic determination of cholesterol. J. Biochem. Biophy. Methods 3843-52. - PubMed
-
- Anderson, F. C., R. Lira, S. Kamhawi, Y. Belkaid, T. A. Wynn, and D. Sacks. 2008. Il-10 and TGF-β control the establishment of persistent and transmissible infections produced by Leishmania tropica in C57BL/6 mice. J. Immunol. 1804090-4097. - PubMed
-
- Anderson, H. A., E. M. Hiltbold, and P. A. Roche. 2000. Concentration of MHC class II molecules in lipid rafts facilitates antigen presentation. Nat. Immunol. 1156-162. - PubMed
-
- Banerjee, A., M. De, and N. Ali. 2008. Complete cure of experimental visceral leishmanisis with amphotericin B in stearylamine-bearing cationic liposomes involves down-regulation of IL-10 and favourable T cell responses. J. Immunol. 1811386-1398. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

