mu-Opioid receptor cell surface expression is regulated by its direct interaction with Ribophorin I
- PMID: 19289571
- PMCID: PMC2684882
- DOI: 10.1124/mol.108.054064
mu-Opioid receptor cell surface expression is regulated by its direct interaction with Ribophorin I
Abstract
The trafficking of the mu-opioid receptor (MOR), a member of the rhodopsin G protein-coupled receptor (GPCR) family, can be regulated by interaction with multiple cellular proteins. To determine the proteins involved in receptor trafficking, using the targeted proteomic approach and mass spectrometry analysis, we have identified that Ribophorin I (RPNI), a component of the oligosaccharide transferase complex, could directly interact with MOR. RPNI can be shown to participate in MOR export by the intracellular retention of the receptor after small interfering RNA knockdown of endogenous RPNI. Overexpression of RPNI rescued the surface expression of the MOR 344KFCTR348 deletion mutant independent of calnexin. Furthermore, RPNI regulation of MOR trafficking is dependent on the glycosylation state of the receptor, as reflected by the inability of overexpression of RPNI to affect the trafficking of the N-glycosylation-deficient mutants, or GPCRs that have minimal glycosylation sites. Hence, this novel RPNI chaperone activity is a consequence of N-glycosylation-dependent direct interaction with MOR.
Figures

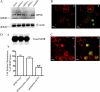
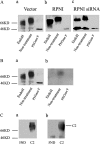
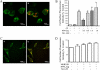

 ,
and
,
and  bars represent the
averages ± S.E.M. in three separated experiments carried out in
triplicate in the absence and presence of RPNI overexpression or treatment
with 1 μM naloxone, respectively; **, p < 0.01
compared with cells transfected with vector.
bars represent the
averages ± S.E.M. in three separated experiments carried out in
triplicate in the absence and presence of RPNI overexpression or treatment
with 1 μM naloxone, respectively; **, p < 0.01
compared with cells transfected with vector.

Similar articles
-
GRIN1 regulates micro-opioid receptor activities by tethering the receptor and G protein in the lipid raft.J Biol Chem. 2009 Dec 25;284(52):36521-36534. doi: 10.1074/jbc.M109.024109. Epub 2009 Oct 27. J Biol Chem. 2009. PMID: 19861419 Free PMC article.
-
MOR is not enough: identification of novel mu-opioid receptor interacting proteins using traditional and modified membrane yeast two-hybrid screens.PLoS One. 2013 Jun 28;8(6):e67608. doi: 10.1371/journal.pone.0067608. Print 2013. PLoS One. 2013. PMID: 23840749 Free PMC article.
-
Activity of the delta-opioid receptor is partially reduced, whereas activity of the kappa-receptor is maintained in mice lacking the mu-receptor.J Neurosci. 1998 Sep 15;18(18):7285-95. doi: 10.1523/JNEUROSCI.18-18-07285.1998. J Neurosci. 1998. PMID: 9736649 Free PMC article.
-
Opioid μ-receptors as new target for insulin resistance.Pharmacol Ther. 2013 Sep;139(3):334-40. doi: 10.1016/j.pharmthera.2013.05.002. Epub 2013 May 18. Pharmacol Ther. 2013. PMID: 23688574 Review.
-
Opioid receptor types and subtypes: the delta receptor as a model.Annu Rev Pharmacol Toxicol. 1996;36:379-401. doi: 10.1146/annurev.pa.36.040196.002115. Annu Rev Pharmacol Toxicol. 1996. PMID: 8725395 Review.
Cited by
-
Species sequence differences determine the interaction of GnRH receptor with the cellular quality control system.Mol Cell Endocrinol. 2013 Dec 5;381(1-2):1-7. doi: 10.1016/j.mce.2013.07.012. Epub 2013 Jul 24. Mol Cell Endocrinol. 2013. PMID: 23891857 Free PMC article.
-
Post-translational Modifications of Opioid Receptors.Trends Neurosci. 2020 Jun;43(6):417-432. doi: 10.1016/j.tins.2020.03.011. Epub 2020 Apr 16. Trends Neurosci. 2020. PMID: 32459993 Free PMC article. Review.
-
GPCR stabilization using the bicelle-like architecture of mixed sterol-detergent micelles.Methods. 2011 Dec;55(4):310-7. doi: 10.1016/j.ymeth.2011.10.011. Epub 2011 Oct 21. Methods. 2011. PMID: 22041719 Free PMC article.
-
Opioid receptors: toward separation of analgesic from undesirable effects.Trends Biochem Sci. 2013 Jun;38(6):275-82. doi: 10.1016/j.tibs.2013.03.003. Epub 2013 Apr 16. Trends Biochem Sci. 2013. PMID: 23598157 Free PMC article. Review.
-
GRIN1 regulates micro-opioid receptor activities by tethering the receptor and G protein in the lipid raft.J Biol Chem. 2009 Dec 25;284(52):36521-36534. doi: 10.1074/jbc.M109.024109. Epub 2009 Oct 27. J Biol Chem. 2009. PMID: 19861419 Free PMC article.
References
-
- Abeijon C and Hirschberg CB (1992) Topography of glycosylation reactions in the endoplasmic reticulum. Trends Biochem Sci 17 32-36. - PubMed
-
- Alder NN and Johnson AE (2004) Cotranslational membrane protein biogenesis at the endoplasmic reticulum. J Biol Chem 279 22787-22790. - PubMed
-
- Bergman LW and Kuehl WM (1979a) Co-translational modification of nascent immunoglobulin heavy and light chains. J Supramol Struct 11 9-24. - PubMed
-
- Bergman LW and Kuehl WM (1979b) Formation of intermolecular disulfide bonds on nascent immunoglobulin polypeptides. J Biol Chem 254 5690-5694. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

