GSK3beta phosphorylation modulates CLASP-microtubule association and lamella microtubule attachment
- PMID: 19289791
- PMCID: PMC2699158
- DOI: 10.1083/jcb.200901042
GSK3beta phosphorylation modulates CLASP-microtubule association and lamella microtubule attachment
Abstract
Polarity of the microtubule (MT) cytoskeleton is essential for many cell functions. Cytoplasmic linker-associated proteins (CLASPs) are MT-associated proteins thought to organize intracellular MTs and display a unique spatiotemporal regulation. In migrating epithelial cells, CLASPs track MT plus ends in the cell body but bind along MTs in the lamella. In this study, we demonstrate that glycogen synthase kinase 3beta (GSK3beta) directly phosphorylates CLASPs at multiple sites in the domain required for MT plus end tracking. Although complete phosphorylation disrupts both plus end tracking and association along lamella MTs, we show that partial phosphorylation of the identified GSK3beta motifs determines whether CLASPs track plus ends or associate along MTs. In addition, we find that expression of constitutively active GSK3beta destabilizes lamella MTs by disrupting lateral MT interactions with the cell cortex. GSK3beta-induced lamella MT destabilization was partially rescued by expression of CLASP2 with mutated phosphorylation sites. This indicates that CLASP-mediated stabilization of peripheral MTs, which likely occurs in the vicinity of focal adhesions, may be regulated by local GSK3beta inactivation.
Figures
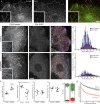

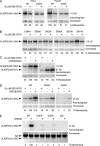



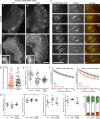
References
-
- Akhmanova A., Steinmetz M.O. 2008. Tracking the ends: a dynamic protein network controls the fate of microtubule tips.Nat. Rev. Mol. Cell Biol. 9:309–322 - PubMed
-
- Akhmanova A., Hoogenraad C.C., Drabek K., Stepanova T., Dortland B., Verkerk T., Vermeulen W., Burgering B.M., De Zeeuw C.I., Grosveld F., Galjart N. 2001. Clasps are CLIP-115 and -170 associating proteins involved in the regional regulation of microtubule dynamics in motile fibroblasts.Cell. 104:923–935 - PubMed
-
- Cassimeris L., Spittle C. 2001. Regulation of microtubule-associated proteins.Int. Rev. Cytol. 210:163–226 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

