Wac: a new Augmin subunit required for chromosome alignment but not for acentrosomal microtubule assembly in female meiosis
- PMID: 19289792
- PMCID: PMC2699157
- DOI: 10.1083/jcb.200811102
Wac: a new Augmin subunit required for chromosome alignment but not for acentrosomal microtubule assembly in female meiosis
Abstract
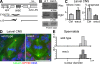
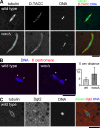
The bipolar spindle forms without centrosomes naturally in female meiosis and by experimental manipulation in mitosis. Augmin is a recently discovered protein complex required for centrosome-independent microtubule generation within the spindle in Drosophila melanogaster cultured cells. Five subunits of Augmin have been identified so far, but neither their organization within the complex nor their role in developing organisms is known. In this study, we report a new Augmin subunit, wee Augmin component (Wac). Wac directly interacts with another Augmin subunit, Dgt2, via its coiled-coil domain. Wac depletion in cultured cells, especially without functional centrosomes, causes severe defects in spindle assembly. We found that a wac deletion mutant is viable but female sterile and shows only a mild impact on somatic mitosis. Unexpectedly, mutant female meiosis showed robust microtubule assembly of the acentrosomal spindle but frequent chromosome misalignment. For the first time, this study establishes the role of an Augmin subunit in developing organisms and provides an insight into the architecture of the complex.
Figures



References
-
- Ashburner M., Golic K.G., Hawley R.S. 2005. Drosophila: A Laboratory Handbook. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY: 1409
-
- Basto R., Lau J., Vinogradova T., Gardiol A., Woods C.G., Khodjakov A., Raff J.W. 2006. Flies without centrioles.Cell. 125:1375–1386 - PubMed
-
- Cullen C.F., Ohkura H. 2001. Msps protein is localized to acentrosomal poles to ensure bipolarity of Drosophila meiotic spindles.Nat. Cell Biol. 3:637–642 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

