Seizures, sensorineural deafness, ataxia, mental retardation, and electrolyte imbalance (SeSAME syndrome) caused by mutations in KCNJ10
- PMID: 19289823
- PMCID: PMC2656559
- DOI: 10.1073/pnas.0901749106
Seizures, sensorineural deafness, ataxia, mental retardation, and electrolyte imbalance (SeSAME syndrome) caused by mutations in KCNJ10
Abstract
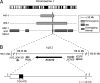
We describe members of 4 kindreds with a previously unrecognized syndrome characterized by seizures, sensorineural deafness, ataxia, mental retardation, and electrolyte imbalance (hypokalemia, metabolic alkalosis, and hypomagnesemia). By analysis of linkage we localize the putative causative gene to a 2.5-Mb segment of chromosome 1q23.2-23.3. Direct DNA sequencing of KCNJ10, which encodes an inwardly rectifying K(+) channel, identifies previously unidentified missense or nonsense mutations on both alleles in all affected subjects. These mutations alter highly conserved amino acids and are absent among control chromosomes. Many of these mutations have been shown to cause loss of function in related K(+) channels. These findings demonstrate that loss-of-function mutations in KCNJ10 cause this syndrome, which we name SeSAME. KCNJ10 is expressed in glia in the brain and spinal cord, where it is believed to take up K(+) released by neuronal repolarization, in cochlea, where it is involved in the generation of endolymph, and on the basolateral membrane in the distal nephron. We propose that KCNJ10 is required in the kidney for normal salt reabsorption in the distal convoluted tubule because of the need for K(+) recycling across the basolateral membrane to enable normal activity of the Na(+)-K(+)-ATPase; loss of this function accounts for the observed electrolyte defects. Mice deficient for KCNJ10 show a related phenotype with seizures, ataxia, and hearing loss, further supporting KCNJ10's role in this syndrome. These findings define a unique human syndrome, and establish the essential role of basolateral K(+) channels in renal electrolyte homeostasis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Karet FE, et al. Mutations in the gene encoding B1 subunit of H+-ATPase cause renal tubular acidosis with sensorineural deafness. Nat Genet. 1999;21:84–90. - PubMed
-
- Birkenhager R, et al. Mutation of BSND causes Bartter syndrome with sensorineural deafness and kidney failure. Nat Genet. 2001;29:310–314. - PubMed
-
- Hansson JH, et al. Hypertension caused by a truncated epithelial sodium channel gamma subunit: genetic heterogeneity of Liddle syndrome. Nat Genet. 1995;11:76–82. - PubMed
-
- Simon DB, et al. Mutations in the chloride channel gene, CLCNKB, cause Bartter's syndrome type III. Nat Genet. 1997;17:171–178. - PubMed
-
- Simon DB, et al. Bartter's syndrome, hypokalaemic alkalosis with hypercalciuria, is caused by mutations in the Na-K-2Cl cotransporter NKCC2. Nat Genet. 1996;13:183–188. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

