The two-component system QseEF and the membrane protein QseG link adrenergic and stress sensing to bacterial pathogenesis
- PMID: 19289831
- PMCID: PMC2667056
- DOI: 10.1073/pnas.0811409106
The two-component system QseEF and the membrane protein QseG link adrenergic and stress sensing to bacterial pathogenesis
Abstract
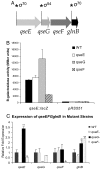
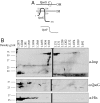
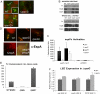
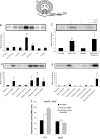
Bacterial pathogens sense host cues to activate expression of virulence genes. Most of these signals are sensed through histidine kinases (HKs), which comprise the main sensory mechanism in bacteria. The host stress hormones epinephrine (Epi) and norepinephrine are sensed through the QseC HK, which initiates a complex signaling cascade to regulate virulence gene expression in enterohemorrhagic Escherichia coli (EHEC). Epi signaling through QseC activates expression of the genes encoding the QseEF 2-component system. QseE is an HK, and QseF is a response regulator. Here, we show that QseE is a second bacterial adrenergic receptor that gauges the stress signals Epi, sulfate, and phosphate. The qseEF genes are organized within an unusual operonic structure, in that a gene is encoded between qseE and qseF. This gene was renamed qseG, and it was shown to encode an outer membrane (OM) protein. EHEC uses a type III secretion system (TTSS) to translocate effector proteins to the epithelial cells that rearrange the host cytoskeleton to form pedestal-like structures that cup the bacterium. QseE, QseG, and QseF are necessary for pedestal formation. Although QseE and QseF are involved in the transcriptional control of genes necessary for pedestal formation, QseG is necessary for translocation of effectors into epithelial cells. QseG is an OM protein necessary for translocation of TTSS effectors that also works in conjunction with a 2-component signaling system that senses host stress signals.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Bearson BL, Bearson SM. The role of the QseC quorum-sensing sensor kinase in colonization and norepinephrine-enhanced motility of Salmonella enterica serovar Typhimurium. Microb Pathog. 2007;44:271–278. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

