Galactose oxidase as a model for reactivity at a copper superoxide center
- PMID: 19290629
- PMCID: PMC2683747
- DOI: 10.1021/ja807963e
Galactose oxidase as a model for reactivity at a copper superoxide center
Abstract
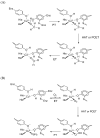
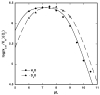
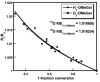
The mononuclear copper enzyme, galactose oxidase, has been investigated under steady-state conditions via O(2)-consumption assays using 1-O-methyl-alpha-D-galactopyranoside as the sugar substrate to produce an aldehyde at the C-6 position. The rate-determining step of the oxidative half-reaction was probed through the measurement of substrate and solvent deuterium and O-18 isotope effects on k(cat)/K(m)(O(2)). The reaction conforms to a ping-pong mechanism with the kinetic parameters for the reductive half, k(cat)/K(m)(S) = 8.3 x 10(3) M(-1) s(-1) at 10 degrees C and pH 7.0, comparing favorably to literature values. The oxidative half-reaction yielded a value of k(cat)/K(m)(O(2)) = 2.5 x 10(6) M(-1) s(-1). A substrate deuterium isotope effect of 32 was measured for the k(cat)/K(m)(S), while a smaller, but significant value of 1.6-1.9 was observed on k(cat)/K(m)(O(2)). O-18 isotope effects of 1.0185 with either protiated or deuterated sugar, together with the absence of any solvent isotope effect, lead to the conclusion that hydrogen atom transfer from reduced cofactor to a Cu(II)-superoxo intermediate is fully rate-determining for k(cat)/K(m)(O(2)). The measured O-18 isotope effects provide corroborative evidence for the reactive superoxo species in the dopamine beta-monooxygenase/peptidylglycine alpha-hydroxylating monooxygenase family, as well as providing a frame of reference for copper-superoxo reactivity. The combination of solvent and substrate deuterium isotope effects rules out solvent deuterium exchange into reduced enzyme as the origin of the relatively small substrate deuterium isotope effect on k(cat)/K(m)(O(2)). These data indicate fundamental differences in the hydrogen transfer step from the carbon of substrate vs the oxygen of reduced cofactor during the reductive and oxidative half-reactions of galactose oxidase.
Figures


References
-
- Rogers MS, Baron AJ, McPherson MJ, Knowles PF, Dooley DM. J. Am. Chem. Soc. 2000;122:990–991.
-
- Whittaker MM, Whittaker JW. J. Biol. Chem. 2003;278:22090–22101. - PubMed
-
- Whittaker MM, Ballou DP, Whittaker JW. Biochemistry. 1998;37:8426–8436. - PubMed
-
- Borman CD, Saysell CG, Sykes AG. J. Biol. Inorg. Chem. 1997;2:480–487. - PubMed
-
- Klinman JP. Chem. Rev. 1996;96:2541–2561. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

