Absence of transverse tubules contributes to non-uniform Ca(2+) wavefronts in mouse and human embryonic stem cell-derived cardiomyocytes
- PMID: 19290776
- PMCID: PMC3139544
- DOI: 10.1089/scd.2009.0052
Absence of transverse tubules contributes to non-uniform Ca(2+) wavefronts in mouse and human embryonic stem cell-derived cardiomyocytes
Abstract
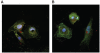
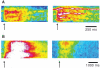
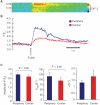
Mouse (m) and human embryonic stem cell-derived cardiomyocytes (hESC-CMs) are known to exhibit immature Ca(2+) dynamics such as small whole-cell peak amplitude and slower kinetics relative to those of adult. In this study, we examined the maturity and efficiency of Ca(2+)-induced Ca(2+) release in m and hESC-CMs, the presence of transverse (t) tubules and its effects on the regional Ca(2+) dynamics. In m and hESC-CMs, fluorescent staining and atomic force microscopy (AFM) were used to detect the presence of t-tubules, caveolin-3, amphiphysin-2 and colocalization of dihydropyridine receptors (DHPRs) and ryanodine receptors (RyRs). To avoid ambiguities, regional electrically-stimulated Ca(2+) dynamics of single ESC-CMs, rather than spontaneously beating clusters, were measured using confocal microscopy. m and hESC-CMs showed absence of dyads, with neither t-tubules nor colocalization of DHPRs and RyRs. Caveolin-3 and amphiphysin-2, crucial for the biogenesis of t-tubules with robust expression in adult CMs, were also absent. Single m and hESC-CMs displayed non-uniform Ca(2+) dynamics across the cell that is typical of CMs deficient of t-tubules. Local Ca(2+) transients exhibited greater peak amplitude at the peripheral than at the central region for m (3.50 +/- 0.42 vs. 3.05 +/- 0.38) and hESC-CMs (2.96 +/- 0.25 vs. 2.72 +/- 0.25). Kinetically, both the rates of rise to peak amplitude and transient decay were faster for the peripheral relative to the central region. Immature m and hESC-CMs display unsynchronized Ca(2+) transients due to the absence of t-tubules and gene products crucial for their biogenesis. Our results provide insights for driving the maturation of ESC-CMs.
Figures



References
-
- Liu J, Fu JD, Siu CW, Li RA. Functional sarcoplasmic reticulum for calcium-handling of human embryonic stem cell-derived cardiomyocytes: insights for driven maturation. Stem Cells. (2007);25:3038–3044. - PubMed
-
- Sartiani L, Bettiol E, Stillitano F, Mugelli A, Cerbai E, Jaconi ME. Developmental changes in cardiomyocytes differentiated from human embryonic stem cells: a molecular and electrophysiological approach. Stem Cells. (2007);25:1136–1144. - PubMed
-
- Itzhaki I, Schiller J, Beyar R, Satin J, Gepstein L. Calcium handling in embryonic stem cell-derived cardiac myocytes: of mice and men. Ann N Y Acad Sci. (2006);1080:207–215. - PubMed
-
- Bers DM. Cardiac excitation–contraction coupling. Nature. (2002);415:198–205. - PubMed
-
- Brette F, Orchard C. T-tubule function in mammalian cardiac myocytes. Circ Res. (2003);92:1182–1192. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

