The tyrosine kinase network regulating mast cell activation
- PMID: 19290926
- PMCID: PMC2669301
- DOI: 10.1111/j.1600-065X.2008.00742.x
The tyrosine kinase network regulating mast cell activation
Abstract
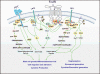
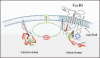
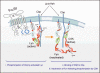
Mast cell mediator release represents a pivotal event in the initiation of inflammatory reactions associated with allergic disorders. These responses follow antigen-mediated aggregation of immunoglobulin E (IgE)-occupied high-affinity receptors for IgE (Fc epsilon RI) on the mast cell surface, a response which can be further enhanced following stem cell factor-induced ligation of the mast cell growth factor receptor KIT (CD117). Activation of tyrosine kinases is central to the ability of both Fc epsilon RI and KIT to transmit downstream signaling events required for the regulation of mast cell activation. Whereas KIT possesses inherent tyrosine kinase activity, Fc epsilon RI requires the recruitment of Src family tyrosine kinases and Syk to control the early receptor-proximal signaling events. The signaling pathways propagated by these tyrosine kinases can be further upregulated by the Tec kinase Bruton's tyrosine kinase and downregulated by the actions of the tyrosine Src homology 2 domain-containing phosphatase 1 (SHP-1) and SHP-2. In this review, we discuss the regulation and role of specific members of this tyrosine kinase network in KIT and Fc epsilon RI-mediated mast cell activation.
Figures


References
-
- Kirshenbaum AS, et al. Demonstration that human mast cells arise from a progenitor cell population that is CD34+, c-kit+, and expresses aminopeptidase N (CD13) Blood. 1999;94:2333–2342. - PubMed
-
- Marshall JS. Mast-cell responses to pathogens. Nat Rev Immunol. 2004;4:787–799. - PubMed
-
- Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat Immunol. 2005;6:135–142. - PubMed
-
- Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997;77:1033–1079. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

