Intralesional administration of epidermal growth factor-based formulation (Heberprot-P) in chronic diabetic foot ulcer: treatment up to complete wound closure
- PMID: 19291119
- PMCID: PMC7951202
- DOI: 10.1111/j.1742-481X.2008.00561.x
Intralesional administration of epidermal growth factor-based formulation (Heberprot-P) in chronic diabetic foot ulcer: treatment up to complete wound closure
Abstract
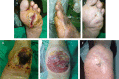
Previous studies have shown that an epidermal growth factor-based formulation (Heberprot-P) can enhance granulation of high-grade diabetic foot ulcers (DFU). The aim of this study was to explore the clinical effects of this administration up to complete wound closure. A pilot study in 20 diabetic patients with full-thickness lower extremity ulcers of more than 4 weeks of evolution was performed. Mean ulcer size was 16.3 +/- 21.3 cm(2). Intralesional injections of 75 microg of Heberprot-P three times per week were given up to complete wound healing. Full granulation response was achieved in all 20 patients in 23.6 +/- 3.8 days. Complete wound closure was obtained in 17 (85%) cases in 44.3 +/- 8.9 days. Amputation was not necessary in any case and only one relapse was notified. The most frequent adverse events were tremors, chills, pain and odour at site of administration and local infection. The therapeutic scheme of intralesional Heberprot-P administration up to complete closure can be safe and suitable to improve the therapeutic goal in terms of healing of chronic DFU.
Figures
References
-
- Frykberg RG, Zgonis T, Armstrong DG, Driver VR, Giurini JM, Kravitz SR, Landsman AS, Lavery LA, Moore JC, Schuberth JM, Wukich DK, Andersen C, Vanore JV. American College of Foot and Ankle Surgeons. Diabetic foot disorders. A clinical practice guideline (2006 revision). J Foot Ankle Surg 2006;45(5 Suppl):S1–S66. - PubMed
-
- Abbott CA, Carrington AL, Ashe H, Bath S, Every LC, Griffiths J, Hann AW, Hussein A, Jackson N, Johnson KE, Ryder CH, Torkington R, Van Ross ERE, Whalley AM, Widdows P, Williamson S, Boulton AJM. The North‐West Diabetes Foot Care Study: incidence of, and risk factors for, new diabetic foot ulceration in a community‐based patient cohort. Diabet Med 2002;19:377–84. - PubMed
-
- Abbott CA, Vileikyte L, Williamson S, Carrington AL, Boulton AJM. Multicenter study of the incidence of and predictive risk factors for diabetic neuropathic foot ulceration. Diabetes Care 1998;21:1071–5. - PubMed
-
- Boulton AJ, Vileikyte L, Ragnarson‐Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet 2005;366:1719–24. - PubMed
-
- Wieman TJ, Smiell JM, Su Y. Efficacy and safety of a topical gel formulation of recombinant human platelet‐derived growth factor‐BB (becaplermin) in patients with chronic neuropathic diabetic ulcers. A phase III randomized placebo‐controlled double‐blind study. Diabetes Care 1998;21:822–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

