Elevated P75NTR expression causes death of engrailed-deficient midbrain dopaminergic neurons by Erk1/2 suppression
- PMID: 19291307
- PMCID: PMC2667502
- DOI: 10.1186/1749-8104-4-11
Elevated P75NTR expression causes death of engrailed-deficient midbrain dopaminergic neurons by Erk1/2 suppression
Abstract
Background: The homeodomain transcription factors Engrailed-1 and Engrailed-2 are required for the survival of mesencephalic dopaminergic (mesDA) neurons in a cell-autonomous and gene-dose-dependent manner. Homozygote mutant mice, deficient of both genes (En1-/-;En2-/-), die at birth and exhibit a loss of all mesDA neurons by mid-gestation. In heterozygote animals (En1+/-;En2-/-), which are viable and fertile, postnatal maintenance of the nigrostriatal dopaminergic system is afflicted, leading to a progressive degeneration specific to this subpopulation and Parkinson's disease-like molecular and behavioral deficits.
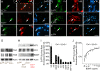
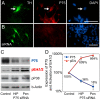
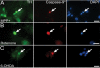
Results: In this work, we show that the dose of Engrailed is inversely correlated to the expression level of the pan-neurotrophin receptor gene P75NTR (Ngfr). Loss of mesDA neurons in the Engrailed-null mutant embryos is caused by elevated expression of this neurotrophin receptor: Unusually, in this case, the cell death signal of P75NTR is mediated by suppression of Erk1/2 (extracellular-signal-regulated kinase 1/2) activity. The reduction in expression of Engrailed, possibly related to the higher levels of P75NTR, also decreases mitochondrial stability. In particular, the dose of Engrailed determines the sensitivity to cell death induced by the classic Parkinson-model toxin MPTP and to inhibition of the anti-apoptotic members of the Bcl-2 family of proteins.
Conclusion: Our study links the survival function of the Engrailed genes in developing mesDA neurons to the regulation of P75NTR and the sensitivity of these neurons to mitochondrial insult. The similarities to the disease etiology in combination with the nigral phenotype of En1+/-;En2-/- mice suggests that haplotype variations in the Engrailed genes and/or P75NTR that alter their expression levels could, in part, determine susceptibility to Parkinson's disease.
Figures



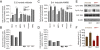
Similar articles
-
Slow progressive degeneration of nigral dopaminergic neurons in postnatal Engrailed mutant mice.Proc Natl Acad Sci U S A. 2006 Oct 10;103(41):15242-7. doi: 10.1073/pnas.0602116103. Epub 2006 Oct 2. Proc Natl Acad Sci U S A. 2006. PMID: 17015829 Free PMC article.
-
Midbrain dopaminergic neurons: control of their cell fate by the engrailed transcription factors.Cell Tissue Res. 2004 Oct;318(1):53-61. doi: 10.1007/s00441-004-0973-8. Epub 2004 Sep 1. Cell Tissue Res. 2004. PMID: 15340832 Review.
-
Fate of midbrain dopaminergic neurons controlled by the engrailed genes.J Neurosci. 2001 May 1;21(9):3126-34. doi: 10.1523/JNEUROSCI.21-09-03126.2001. J Neurosci. 2001. PMID: 11312297 Free PMC article.
-
Characterization of the Engrailed mutant mice as experimental models for Parkinson's disease.Parkinsonism Relat Disord. 2008;14 Suppl 2:S103-6. doi: 10.1016/j.parkreldis.2008.04.011. Epub 2008 Jun 27. Parkinsonism Relat Disord. 2008. PMID: 18585948
-
Progressive loss of dopaminergic neurons in the ventral midbrain of adult mice heterozygote for Engrailed1: a new genetic model for Parkinson's disease?Parkinsonism Relat Disord. 2008;14 Suppl 2:S107-11. doi: 10.1016/j.parkreldis.2008.04.007. Epub 2008 Jun 27. Parkinsonism Relat Disord. 2008. PMID: 18585951 Review.
Cited by
-
The transcription factor Pax6 regulates survival of dopaminergic olfactory bulb neurons via crystallin αA.Neuron. 2010 Nov 18;68(4):682-94. doi: 10.1016/j.neuron.2010.09.030. Neuron. 2010. PMID: 21092858 Free PMC article.
-
Induced neural stem cells protect neuronal cells against apoptosis.Med Sci Monit. 2014 Dec 22;20:2759-66. doi: 10.12659/MSM.891343. Med Sci Monit. 2014. PMID: 25554259 Free PMC article.
-
Neuroprotective Transcription Factors in Animal Models of Parkinson Disease.Neural Plast. 2016;2016:6097107. doi: 10.1155/2016/6097107. Epub 2015 Dec 31. Neural Plast. 2016. PMID: 26881122 Free PMC article. Review.
-
Loss of GABAergic neurons in the hippocampus and cerebral cortex of Engrailed-2 null mutant mice: implications for autism spectrum disorders.Exp Neurol. 2013 Sep;247:496-505. doi: 10.1016/j.expneurol.2013.01.021. Epub 2013 Jan 27. Exp Neurol. 2013. PMID: 23360806 Free PMC article.
-
Oxidative Stress Suppresses Trk Signaling While Stimulating JNK-Mediated Endocytosis and Cleavage of p75NTR: A Targetable Pathway for Neuroprotection in a Parkinson's Disease Model.J Neurochem. 2025 Feb;169(2):e70010. doi: 10.1111/jnc.70010. J Neurochem. 2025. PMID: 39936238
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

