The PI3K-Akt-mTOR pathway regulates Abeta oligomer induced neuronal cell cycle events
- PMID: 19291319
- PMCID: PMC2663563
- DOI: 10.1186/1750-1326-4-14
The PI3K-Akt-mTOR pathway regulates Abeta oligomer induced neuronal cell cycle events
Abstract
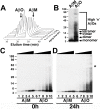
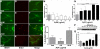
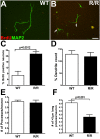
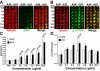
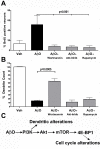
Accumulating evidence suggests that neurons prone to degeneration in Alzheimer's Disease (AD) exhibit evidence of re-entry into an aberrant mitotic cell cycle. Our laboratory recently demonstrated that, in a genomic amyloid precursor protein (APP) mouse model of AD (R1.40), neuronal cell cycle events (CCEs) occur in the absence of beta-amyloid (Abeta) deposition and are still dependent upon the amyloidogenic processing of the amyloid precursor protein (APP). These data suggested that soluble Abeta species might play a direct role in the induction of neuronal CCEs. Here, we show that exposure of non-transgenic primary cortical neurons to Abeta oligomers, but not monomers or fibrils, results in the retraction of neuronal processes, and induction of CCEs in a concentration dependent manner. Retraction of neuronal processes correlated with the induction of CCEs and the Abeta monomer or Abeta fibrils showed only minimal effects. In addition, we provide evidence that induction of neuronal CCEs are autonomous to primary neurons cultured from the R1.40 mice. Finally, our results also demonstrate that Abeta oligomer treated neurons exhibit elevated levels of activated Akt and mTOR (mammalian Target Of Rapamycin) and that PI3K, Akt or mTOR inhibitors blocked Abeta oligomer-induced neuronal CCEs. Taken together, these results demonstrate that Abeta oligomer-based induction of neuronal CCEs involve the PI3K-Akt-mTOR pathway.
Figures



References
-
- Selkoe DJ. Alzheimer's disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. - PubMed
-
- Copani A, Caraci F, Hoozemans JJ, Calafiore M, Sortino MA, Nicoletti F. The nature of the cell cycle in neurons: focus on a "non-canonical" pathway of DNA replication causally related to death. Biochim Biophys Acta. 2007;1772:409–412. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

