Biosynthesis of aromatic polyketides in bacteria
- PMID: 19292437
- PMCID: PMC2696626
- DOI: 10.1021/ar8002249
Biosynthesis of aromatic polyketides in bacteria
Abstract
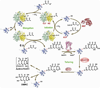
Natural products, produced chiefly by microorganisms and plants, can be large and structurally complex molecules. These molecules are manufactured by cellular assembly lines, in which enzymes construct the molecules in a stepwise fashion. The means by which enzymes interact and work together in a modular fashion to create diverse structural features has been an active area of research; the work has provided insight into the fine details of biosynthesis. A number of polycyclic aromatic natural products--including several noteworthy anticancer, antibacterial, antifungal, antiviral, antiparasitic, and other medicinally significant substances--are synthesized by polyketide synthases (PKSs) in soil-borne bacteria called actinomycetes. Concerted biosynthetic, enzymological, and structural biological investigations into these modular enzyme systems have yielded interesting mechanistic insights. A core module called the minimal PKS is responsible for synthesizing a highly reactive, protein-bound poly-beta-ketothioester chain. In the absence of other enzymes, the minimal PKS also catalyzes chain initiation and release, yielding an assortment of polycyclic aromatic compounds. In the presence of an initiation PKS module, polyketide backbones bearing additional alkyl, alkenyl, or aryl primer units are synthesized, whereas a range of auxiliary PKS enzymes and tailoring enzymes convert the product of the minimal PKS into the final natural product. In this Account, we summarize the knowledge that has been gained regarding this family of PKSs through recent investigations into the biosynthetic pathways of two natural products, actinorhodin and R1128 (A-D). We also discuss the practical relevance of these fundamental insights for the engineered biosynthesis of new polycyclic aromatic compounds. With a deeper understanding of the biosynthetic process in hand, we can assert control at various stages of molecular construction and thus introduce unnatural functional groups in the process. The metabolic engineer affords a number of new avenues for creating novel molecular structures that will likely have properties akin to their fully natural cousins.
Figures

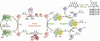
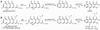
References
-
- O'Hagan D. The Polyketide Metabolites. Ellis Horwood: Chichester; 1991.
-
- Rawlings BJ. Biosynthesis of polyketides (other than actinomycete macrolides) Nat. Prod. Rep. 1999;16:425–484. - PubMed
-
- Richardson M, Khosla C. Structure, Function, and Engineering of Bacterial Aromatic Polyketide Synthases. Comprehensive Natural Products Chemistry. 1999;1:473.
-
- Shen B. Biosynthesis of Aromatic Polyketides. Topics in Current Chemistry. 2000;209:1.
-
- Hertweck C, Luzhetskyy A, Rebets Y, Bechthold A. Type II polyketide synthases: gaining a deeper insight into enzymatic teamwork. Nat. Prod. Rep. 2007;24:162–190. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

