Cholesterol exposure at the membrane surface is necessary and sufficient to trigger perfringolysin O binding
- PMID: 19292457
- PMCID: PMC2825173
- DOI: 10.1021/bi9002309
Cholesterol exposure at the membrane surface is necessary and sufficient to trigger perfringolysin O binding
Abstract
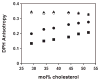
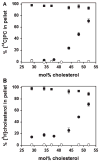
Perfringolysin O (PFO) is the prototype for the cholesterol-dependent cytolysins, a family of bacterial pore-forming toxins that act on eukaryotic membranes. The pore-forming mechanism of PFO exhibits an absolute requirement for membrane cholesterol, but the complex interplay between the structural arrangement of the PFO C-terminal domain and the distribution of cholesterol in the target membrane is poorly understood. Herein we show that PFO binding to the bilayer and the initiation of the sequence of events that culminate in the formation of a transmembrane pore depend on the availability of free cholesterol at the membrane surface, while changes in the acyl chain packing of the phospholipids and cholesterol in the membrane core, or the presence or absence of detergent-resistant domains do not correlate with PFO binding. Moreover, PFO association with the membrane was inhibited by the addition of sphingomyelin, a typical component of membrane rafts in cell membranes. Finally, addition of molecules that do not interact with PFO, but intercalate into the membrane and displace cholesterol from its association with phospholipids (e.g., epicholesterol), reduced the amount of cholesterol required to trigger PFO binding. Taken together, our studies reveal that PFO binding to membranes is triggered when the concentration of cholesterol exceeds the association capacity of the phospholipids, and this cholesterol excess is then free to associate with the toxin.
Figures




Similar articles
-
Perfringolysin O: The Underrated Clostridium perfringens Toxin?Toxins (Basel). 2015 May 14;7(5):1702-21. doi: 10.3390/toxins7051702. Toxins (Basel). 2015. PMID: 26008232 Free PMC article. Review.
-
How interaction of perfringolysin O with membranes is controlled by sterol structure, lipid structure, and physiological low pH: insights into the origin of perfringolysin O-lipid raft interaction.J Biol Chem. 2008 Feb 22;283(8):4632-42. doi: 10.1074/jbc.M709483200. Epub 2007 Dec 17. J Biol Chem. 2008. PMID: 18089559
-
Interaction of Cholesterol with Perfringolysin O: What Have We Learned from Functional Analysis?Toxins (Basel). 2017 Nov 23;9(12):381. doi: 10.3390/toxins9120381. Toxins (Basel). 2017. PMID: 29168745 Free PMC article. Review.
-
R468A mutation in perfringolysin O destabilizes toxin structure and induces membrane fusion.Biochim Biophys Acta Biomembr. 2017 Jun;1859(6):1075-1088. doi: 10.1016/j.bbamem.2017.03.001. Epub 2017 Mar 2. Biochim Biophys Acta Biomembr. 2017. PMID: 28263714
-
Phospholipid hydrolysis caused by Clostridium perfringens α-toxin facilitates the targeting of perfringolysin O to membrane bilayers.Biochemistry. 2010 Nov 9;49(44):9498-507. doi: 10.1021/bi1013886. Biochemistry. 2010. PMID: 20886855
Cited by
-
Perfringolysin O: The Underrated Clostridium perfringens Toxin?Toxins (Basel). 2015 May 14;7(5):1702-21. doi: 10.3390/toxins7051702. Toxins (Basel). 2015. PMID: 26008232 Free PMC article. Review.
-
Identification and characterization of the first cholesterol-dependent cytolysins from Gram-negative bacteria.Infect Immun. 2013 Jan;81(1):216-25. doi: 10.1128/IAI.00927-12. Epub 2012 Oct 31. Infect Immun. 2013. PMID: 23115036 Free PMC article.
-
Single-molecule tracking of perfringolysin O assembly and membrane insertion uncoupling.FEBS J. 2023 Jan;290(2):428-441. doi: 10.1111/febs.16596. Epub 2022 Sep 19. FEBS J. 2023. PMID: 35989549 Free PMC article.
-
Disentangling the roles of cholesterol and CD59 in intermedilysin pore formation.Sci Rep. 2016 Dec 2;6:38446. doi: 10.1038/srep38446. Sci Rep. 2016. PMID: 27910935 Free PMC article.
-
The synergistic necrohemorrhagic action of Clostridium perfringens perfringolysin and alpha toxin in the bovine intestine and against bovine endothelial cells.Vet Res. 2013 Jun 19;44(1):45. doi: 10.1186/1297-9716-44-45. Vet Res. 2013. PMID: 23782465 Free PMC article.
References
-
- Alouf JE, Billington SJ, Jost BH. Repertoire and general features of the family of cholesterol-dependent cytolysins. In: Alouf JE, Popoff MR, editors. The Comprehensive Sourcebook of Bacterial Protein Toxins. Academic Press; Oxford, England: 2005. pp. 643–658.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

