A proximal centriole-like structure is present in Drosophila spermatids and can serve as a model to study centriole duplication
- PMID: 19293139
- PMCID: PMC2674812
- DOI: 10.1534/genetics.109.101709
A proximal centriole-like structure is present in Drosophila spermatids and can serve as a model to study centriole duplication
Abstract
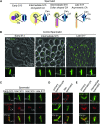
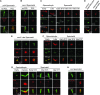
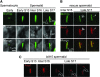
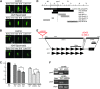
Most animals have two centrioles in spermatids (the distal and proximal centrioles), but insect spermatids seem to contain only one centriole (Fuller 1993), which functionally resembles the distal centriole. Using fluorescent centriolar markers, we identified a structure near the fly distal centriole that is reminiscent of a proximal centriole (i.e., proximal centriole-like, or PCL). We show that the PCL exhibits several features of daughter centrioles. First, a single PCL forms near the proximal segment of the older centriole. Second, the centriolar proteins SAS-6, Ana1, and Bld10p/Cep135 are in the PCL. Third, PCL formation depends on SAK/PLK4 and SAS-6. Using a genetic screen for PCL defect, we identified a mutation in the gene encoding the conserved centriolar protein POC1, which is part of the daughter centriole initiation site (Kilburn et al. 2007) in Tetrahymena. We conclude that the PCL resembles an early intermediate structure of a forming centriole, which may explain why no typical centriolar structure is observed under electron microscopy. We propose that, during the evolution of insects, the proximal centriole was simplified by eliminating the later steps in centriole assembly. The PCL may provide a unique model to study early steps of centriole formation.
Figures

References
-
- Andersen, J. S., C. J. Wilkinson, T. Mayor, P. Mortensen, E. A. Nigg et al., 2003. Proteomic characterization of the human centrosome by protein correlation profiling. Nature 426 570–574. - PubMed
-
- Anderson, W. A., 1967. Cytodifferentiation of spermatozoa in Drosophila melanogaster: the effect of elevated temperature on spermiogenesis. Mol. Gen. Genet. 99 257–273. - PubMed
-
- Avidor-Reiss, T., A. M. Maer, E. Koundakjian, A. Polyanovsky, T. Keil et al., 2004. Decoding cilia function: defining specialized genes required for compartmentalized cilia biogenesis. Cell 117 527–539. - PubMed
-
- Azimzadeh, J., and M. Bornens, 2007. Structure and duplication of the centrosome. J. Cell Sci. 120 2139–2142. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

