The membrane mucin Muc4 inhibits apoptosis induced by multiple insults via ErbB2-dependent and ErbB2-independent mechanisms
- PMID: 19293191
- PMCID: PMC2745945
- DOI: 10.1158/0008-5472.CAN-08-2089
The membrane mucin Muc4 inhibits apoptosis induced by multiple insults via ErbB2-dependent and ErbB2-independent mechanisms
Abstract
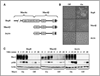
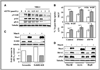
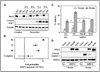
The aberrant expression of membrane mucins such as Muc1 and Muc4 by tumor cells has been shown to engage signaling pathways that promote cellular properties associated with tumor progression. Our previous studies have shown that Muc4 interacts with and potentiates signaling by the ErbB2 (HER2) receptor tyrosine kinase through an epidermal growth factor-like domain in its extracellular region. Here, we show that expression of Muc4 in human A375 melanoma cells and MCF7 breast cancer cells confers resistance to apoptosis induced by a variety of stimuli, including chemotherapeutic agents, the absence of serum factors, and the loss of cellular adhesion. Mapping experiments revealed that the O-glycosylation and cytosolic domains of Muc4 are dispensable for its antiapoptotic activity, and are also dispensable for the potentiation of signaling by ErbB2. Knockdown of endogenous Muc4 in JIMT-1 breast cancer cells sensitizes cells to apoptotic stimuli, and this can be rescued by Muc4 forms lacking the O-glycosylation or cytosolic domains. Surprisingly, however, the molecular mechanisms underlying Muc4 antiapoptotic activity vary among cell lines. Although Muc4 in JIMT-1 cells engages ErbB2 to promote cell survival, its antiapoptotic mechanism in MCF7 and A375 cells seems to be independent of ErbB2. However, Muc4 expression in all cell lines culminates in the phosphorylation and inactivation of the proapoptotic protein Bad and the elevation of the prosurvival protein Bcl-xL. Our observations suggest that tumor cells can exploit the versatile antiapoptotic activities of Muc4 to acquire resistance to therapeutic agents, and augment cell survival after the loss of adhesion and microenvironment-derived survival factors.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures


References
-
- Hattrup CL, Gendler SJ. Structure and function of the cell surface (tethered) mucins. Annu Rev Physiol. 2008;70:431–457. - PubMed
-
- Carraway KL, III, Funes M, Workman HC, Sweeney C. Contribution of membrane mucins to tumor progression through modulation of cellular growth signaling pathways. Curr Top Dev Biol. 2007;78:1–22. - PubMed
-
- Cullen PJ. Signaling mucins: the new kids on the MAPK block. Crit Rev Eukaryot Gene Expr. 2007;17:241–257. - PubMed
-
- Singh PK, Hollingsworth MA. Cell surface-associated mucins in signal transduction. Trends Cell Biol. 2006;16:467–476. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

