Mitochondrial changes within axons in multiple sclerosis
- PMID: 19293237
- PMCID: PMC3605917
- DOI: 10.1093/brain/awp046
Mitochondrial changes within axons in multiple sclerosis
Abstract
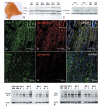
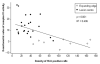
Multiple sclerosis is the most common cause of non-traumatic neurological impairment in young adults. An energy deficient state has been implicated in the degeneration of axons, the pathological correlate of disease progression, in multiple sclerosis. Mitochondria are the most efficient producers of energy and play an important role in calcium homeostasis. We analysed the density and function of mitochondria using immunohistochemistry and histochemistry, respectively, in chronic active and inactive lesions in progressive multiple sclerosis. As shown before in acute pattern III and Balo's lesions, the mitochondrial respiratory chain complex IV activity is reduced despite the presence of mitochondria in demyelinated axons with amyloid precursor protein accumulation, which are predominantly located at the active edge of chronic active lesions. Furthermore, the strong non-phosphorylated neurofilament (SMI32) reactivity was associated with a significant reduction in complex IV activity and mitochondria within demyelinated axons. The complex IV defect associated with axonal injury may be mediated by soluble products of innate immunity, as suggested by an inverse correlation between complex IV activity and macrophage/microglial density in chronic lesions. However, in inactive areas of chronic multiple sclerosis lesions the mitochondrial respiratory chain complex IV activity and mitochondrial mass, judged by porin immunoreactivity, are increased within approximately half of large (>2.5 microm diameter) chronically demyelinated axons compared with large myelinated axons in the brain and spinal cord. The axon-specific mitochondrial docking protein (syntaphilin) and phosphorylated neurofilament-H were increased in chronic lesions. The lack of complex IV activity in a proportion of Na(+)/K(+) ATPase alpha-1 positive demyelinated axons supports axonal dysfunction as a contributor to neurological impairment and disease progression. Furthermore, in vitro studies show that inhibition of complex IV augments glutamate-mediated axonal injury (amyloid precursor protein and SMI32 reactivity). Our findings have important implications for both axonal degeneration and dysfunction during the progressive stage of multiple sclerosis.
Figures

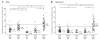




References
-
- Ames A., 3rd CNS energy metabolism as related to function. Brain Res Brain Res Rev. 2000;34:42–68. - PubMed
-
- Andrews H, White K, Thomson C, Edgar J, Bates D, Griffiths I, et al. Increased axonal mitochondrial activity as an adaptation to myelin deficiency in the Shiverer mouse. J Neurosci Res. 2006;83:1533–9. - PubMed
-
- Andreyev AY, Kushnareva YE, Starkov AA. Mitochondrial metabolism of reactive oxygen species. Biochemistry. 2005;70:200–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

