Obesity induces functional astrocytic leptin receptors in hypothalamus
- PMID: 19293246
- PMCID: PMC2668946
- DOI: 10.1093/brain/awp029
Obesity induces functional astrocytic leptin receptors in hypothalamus
Abstract
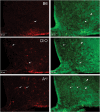
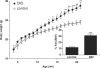
The possible role of astrocytes in the regulation of feeding has been overlooked. It is well-established that the endothelial cells constituting the blood-brain barrier transport leptin from blood to brain and that hypothalamic neurons respond to leptin to induce anorexic signaling. However, few studies have addressed the role of astrocytes in either leptin transport or cellular activation. We recently showed that the obese agouti viable yellow mouse has prominent astrocytic expression of the leptin receptor. In this study, we test the hypothesis that diet-induced obesity increases astrocytic leptin receptor expression and function in the hypothalamus. Double-labelling immunohistochemistry and confocal microscopic analysis showed that all astrocytes in the hypothalamus express leptin receptors. In adult obese mice, 2 months after being placed on a high-fat diet, there was a striking increase of leptin receptor (+) astrocytes, most prominent in the dorsomedial hypothalamus and arcuate nucleus. Agouti viable yellow mice with their adult-onset obesity showed similar changes, but the increase of leptin receptor (+) astrocytes was barely seen in ob/ob or db/db mice with their early-onset obesity and defective leptin systems. The marked leptin receptor protein expression in the astrocytes, shown with several antibodies against different receptor epitopes, was supported by RT-PCR detection of leptin receptor-a and -b mRNAs in primary hypothalamic astrocytes. Unexpectedly, the protein expression of GFAP, a marker of astrocytes, was also increased in adult-onset obesity. Real-time confocal imaging showed that leptin caused a robust increase of calcium signalling in primary astrocytes from the hypothalamus, confirming their functionality. The results indicate that metabolic changes in obese mice can rapidly alter leptin receptor expression and astrocytic activity, and that leptin receptor is responsible for leptin-induced calcium signalling in astrocytes. This novel and clinically relevant finding opens new avenues in astrocyte biology.
Figures







References
-
- Abbott NJ. Cellular compositions of the blood-brain barrier (BBB) In: Kastin AJ, Pan W, editors. The Henry Stewart Talk series: the blood-brain barrier. London: Henry Stewart Talks; 2008.
-
- Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. - PubMed
-
- Ahmed M, Shaban Z, Yamaji D, Okamatsu-Ogura Y, Soliman M, Abd EM, et al. Induction of proinflammatory cytokines and caspase-1 by leptin in monocyte/macrophages from holstein cows. J Vet Med Sci. 2007;69:509–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

