Role for malic enzyme, pyruvate carboxylation, and mitochondrial malate import in glucose-stimulated insulin secretion
- PMID: 19293334
- PMCID: PMC2692397
- DOI: 10.1152/ajpendo.90836.2008
Role for malic enzyme, pyruvate carboxylation, and mitochondrial malate import in glucose-stimulated insulin secretion
Abstract
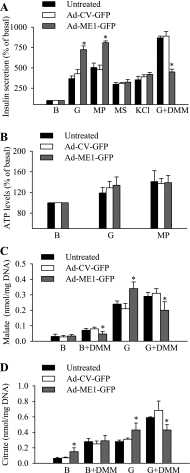
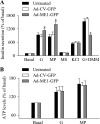
Pyruvate cycling has been implicated in glucose-stimulated insulin secretion (GSIS) from pancreatic beta-cells. The operation of some pyruvate cycling pathways is proposed to necessitate malate export from the mitochondria and NADP(+)-dependent decarboxylation of malate to pyruvate by cytosolic malic enzyme (ME1). Evidence in favor of and against a role of ME1 in GSIS has been presented by others using small interfering RNA-mediated suppression of ME1. ME1 was also proposed to account for methyl succinate-stimulated insulin secretion (MSSIS), which has been hypothesized to occur via succinate entry into the mitochondria in exchange for malate and subsequent malate conversion to pyruvate. In contrast to rat, mouse beta-cells lack ME1 activity, which was suggested to explain their lack of MSSIS. However, this hypothesis was not tested. In this report, we demonstrate that although adenoviral-mediated overexpression of ME1 greatly augments GSIS in rat insulinoma INS-1 832/13 cells, it does not restore MSSIS, nor does it significantly affect GSIS in mouse islets. The increase in GSIS following ME1 overexpression in INS-1 832/13 cells did not alter the ATP-to-ADP ratio but was accompanied by increases in malate and citrate levels. Increased malate and citrate levels were also observed after INS-1 832/13 cells were treated with the malate-permeable analog dimethyl malate. These data suggest that although ME1 overexpression augments anaplerosis and GSIS in INS-1 832/13 cells, it is not likely involved in MSSIS and GSIS in pancreatic islets.
Figures


References
-
- Antinozzi PA, Segall L, Prentki M, McGarry JD, Newgard CB. Molecular or pharmacologic perturbation of the link between glucose and lipid metabolism is without effect on glucose-stimulated insulin secretion. A re-evaluation of the long-chain acyl-CoA hypothesis. J Biol Chem 273: 16146–16154, 1998. - PubMed
-
- Berry MN The function of energy-dependent redox reactions in cell metabolism. FEBS Lett 117 Suppl: K106–K120, 1980. - PubMed
-
- Berry MN, Fanning DC, Grivell AR, Lewis SJ, Farrington CJ, Wallace PG. Evidence for several separate functional pools of NAD(H) within the cytoplasmic compartment of the hepatocyte. Biochem Soc Trans 8: 570, 1980. - PubMed
-
- Boucher A, Lu D, Burgess SC, Telemaque-Potts S, Jensen MV, Mulder H, Wang MY, Unger RH, Sherry AD, Newgard CB. Biochemical mechanism of lipid-induced impairment of glucose-stimulated insulin secretion and reversal with a malate analogue. J Biol Chem 279: 27263–27271, 2004. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

