Activation of critical, host-induced, metabolic and stress pathways marks neutrophil entry into cystic fibrosis lungs
- PMID: 19293384
- PMCID: PMC2667067
- DOI: 10.1073/pnas.0813410106
Activation of critical, host-induced, metabolic and stress pathways marks neutrophil entry into cystic fibrosis lungs
Abstract
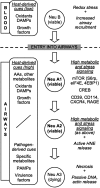
Cystic fibrosis (CF) patients undergo progressive airway destruction caused in part by chronic neutrophilic inflammation. While opportunistic pathogens infecting CF airways can cause inflammation, we hypothesized that host-derived metabolic and stress signals would also play a role in this process. We show that neutrophils that have entered CF airways have increased phosphorylation of the eukaryotic initiation factor 4E and its partner the 4E-binding protein 1; 2 key effectors in the growth factor- and amino acid-regulated mammalian target of rapamycin (mTOR) pathway. Furthermore CF airway neutrophils display increased phosphorylation of the cAMP response element binding protein (CREB), a major transcriptional coactivator in stress signaling cascades. These active intracellular pathways are associated with increased surface expression of critical adaptor molecules, including the growth factor receptor CD114 and the receptor for advanced glycation end-products (RAGE), a CREB inducer and sensor for host-derived damage-associated molecular patterns (DAMPs). Most CF airway fluids lack any detectable soluble RAGE, an inhibitory decoy receptor for DAMPs. Concomitantly, CF airway fluids displayed high and consequently unopposed levels of S100A12; a potent mucosa- and neutrophil-derived DAMP. CF airway neutrophils also show increased surface levels of 2 critical CREB targets, the purine-recycling enzyme CD39 and the multifunctional, mTOR-inducing CXCR4 receptor. This coordinated set of events occurs in all patients, even in the context of minimal airway inflammation and well-preserved lung function. Taken together, our data demonstrate an early and sustained activation of host-responsive metabolic and stress pathways upon neutrophil entry into CF airways, suggesting potential targets for therapeutic modulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Davis PB. Cystic fibrosis since 1938. Am J Respir Crit Care Med. 2006;173:475–482. - PubMed
-
- Tirouvanziam R. Neutrophilic inflammation as a major determinant in the progression of cystic fibrosis. Drug News Perspect. 2006;19:609–614. - PubMed
-
- Meyer KC, Zimmerman J. Neutrophil mediators, pseudomonas, and pulmonary dysfunction in cystic fibrosis. J Lab Clin Med. 1993;121:654–661. - PubMed
-
- Borregaard N, Sorensen OE, Theilgaard-Monch K. Neutrophil granules: A library of innate immunity proteins. Trends Immunol. 2007;28:340–345. - PubMed
-
- McMorran BJ, et al. Novel neutrophil-derived proteins in bronchoalveolar lavage fluid indicate an exaggerated inflammatory response in pediatric cystic fibrosis patients. Clin Chem. 2007;53:1782–1791. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

