Structural plasticity of dentate granule cell mossy fibers during the development of limbic epilepsy
- PMID: 19294647
- PMCID: PMC2801769
- DOI: 10.1002/hipo.20589
Structural plasticity of dentate granule cell mossy fibers during the development of limbic epilepsy
Abstract
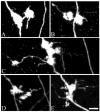
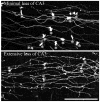
Altered granule cell>>CA3 pyramidal cell synaptic connectivity may contribute to the development of limbic epilepsy. To explore this possibility, granule cell giant mossy fiber bouton plasticity was examined in the kindling and pilocarpine models of epilepsy using green fluorescent protein-expressing transgenic mice. These studies revealed significant increases in the frequency of giant boutons with satellite boutons 2 days and 1 month after pilocarpine status epilepticus, and increases in giant bouton area at 1 month. Similar increases in giant bouton area were observed shortly after kindling. Finally, both models exhibited plasticity of mossy fiber giant bouton filopodia, which contact GABAergic interneurons mediating feedforward inhibition of CA3 pyramids. In the kindling model, however, all changes were fleeting, having resolved by 1 month after the last evoked seizure. Together, these findings demonstrate striking structural plasticity of granule cell mossy fiber synaptic terminal structure in two distinct models of adult limbic epileptogenesis. We suggest that these plasticities modify local connectivities between individual mossy fiber terminals and their targets, inhibitory interneurons, and CA3 pyramidal cells potentially altering the balance of excitation and inhibition during the development of epilepsy.
Copyright 2009 Wiley-Liss, Inc.
Figures







References
-
- Amaral DG. Synaptic extensions from the mossy fibers of the fascia dentata. Anat Embryol (Berl) 1979;155:241–251. - PubMed
-
- Amaral DG, Dent JA. Development of the mossy fibers of the dentate gyrus: I. A light and electron microscopic study of the mossy fibers and their expansions. J Comp Neurol. 1981;195:51–86. - PubMed
-
- André V, Marescaux C, Nehlig A, Fritschy JM. Alterations of hippocampal GAbaergic system contribute to development of spontaneous recurrent seizures in the rat lithium-pilocarpine model of temporal lobe epilepsy. Hippocampus. 2001;11:452–468. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

