Mechanisms of biphasic insulin-granule exocytosis - roles of the cytoskeleton, small GTPases and SNARE proteins
- PMID: 19295123
- PMCID: PMC2720925
- DOI: 10.1242/jcs.034355
Mechanisms of biphasic insulin-granule exocytosis - roles of the cytoskeleton, small GTPases and SNARE proteins
Abstract
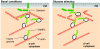
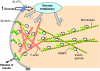
The release of insulin from pancreatic islets requires negative regulation to ensure low levels of insulin release under resting conditions, as well as positive regulation to facilitate robust responsiveness to conditions of elevated fuel or glucose. The first phase of release involves the plasma-membrane fusion of a small pool of granules, termed the readily releasable pool; these granules are already at the membrane under basal conditions, and discharge their cargo in response to nutrient and also non-nutrient secretagogues. By contrast, second-phase secretion is evoked exclusively by nutrients, and involves the mobilization of intracellular granules to t-SNARE sites at the plasma membrane to enable the distal docking and fusion steps of insulin exocytosis. Nearly 40 years ago, the actin cytoskeleton was first recognized as a key mediator of biphasic insulin release, and was originally presumed to act as a barrier to block granule docking at the cell periphery. More recently, however, the discovery of cycling GTPases that are involved in F-actin reorganization in the islet beta-cell, combined with the availability of reagents that are more specific and tools with which to study the mechanisms that underlie granule movement, have contributed greatly to our understanding of the role of the cytoskeleton in regulating biphasic insulin secretion. Herein, we provide historical perspective and review recent progress that has been made towards integrating cytoskeletal reorganization and cycling of small Rho-, Rab- and Ras-family GTPases into our current models of stimulus-secretion coupling and second-phase insulin release.
Figures


Similar articles
-
Emerging Roles of Small GTPases in Islet β-Cell Function.Cells. 2021 Jun 15;10(6):1503. doi: 10.3390/cells10061503. Cells. 2021. PMID: 34203728 Free PMC article. Review.
-
Transduction of MIN6 beta cells with TAT-syntaxin SNARE motif inhibits insulin exocytosis in biphasic insulin release in a distinct mechanism analyzed by evanescent wave microscopy.J Biol Chem. 2002 Dec 27;277(52):50805-11. doi: 10.1074/jbc.M207988200. Epub 2002 Oct 21. J Biol Chem. 2002. PMID: 12393909
-
Recent new insights into the role of SNARE and associated proteins in insulin granule exocytosis.Diabetes Obes Metab. 2017 Sep;19 Suppl 1:115-123. doi: 10.1111/dom.13001. Diabetes Obes Metab. 2017. PMID: 28880475 Review.
-
Insulin granule biogenesis, trafficking and exocytosis.Vitam Horm. 2009;80:473-506. doi: 10.1016/S0083-6729(08)00616-X. Vitam Horm. 2009. PMID: 19251047 Free PMC article. Review.
-
Heterogeneous modes of insulin granule exocytosis: molecular determinants.Front Biosci (Landmark Ed). 2011 Jan 1;16(1):360-7. doi: 10.2741/3692. Front Biosci (Landmark Ed). 2011. PMID: 21196175 Review.
Cited by
-
Changes in Cells Associated with Insulin Resistance.Int J Mol Sci. 2024 Feb 18;25(4):2397. doi: 10.3390/ijms25042397. Int J Mol Sci. 2024. PMID: 38397072 Free PMC article. Review.
-
A Critical Role for β-Catenin in Modulating Levels of Insulin Secretion from β-Cells by Regulating Actin Cytoskeleton and Insulin Vesicle Localization.J Biol Chem. 2016 Dec 9;291(50):25888-25900. doi: 10.1074/jbc.M116.758516. Epub 2016 Oct 24. J Biol Chem. 2016. PMID: 27777306 Free PMC article.
-
Insulin secretion: The nitric oxide controversy.EXCLI J. 2020 Sep 8;19:1227-1245. doi: 10.17179/excli2020-2711. eCollection 2020. EXCLI J. 2020. PMID: 33088259 Free PMC article. Review.
-
Isolation and Proteomics of the Insulin Secretory Granule.Metabolites. 2021 Apr 30;11(5):288. doi: 10.3390/metabo11050288. Metabolites. 2021. PMID: 33946444 Free PMC article. Review.
-
Chronic high-fat diet in fathers programs β-cell dysfunction in female rat offspring.Nature. 2010 Oct 21;467(7318):963-6. doi: 10.1038/nature09491. Nature. 2010. PMID: 20962845
References
-
- Asahara, S., Kido, Y., Shigeyama, T., Matsuda, T., Takeda, A., Inoue, T., Shibutani, Y., Koyanagi, M., Uchida, T. and Kasuga, M. (2008). Rac1 regulates glucose-induced insulin secretion through modulation of cytoskeleton organization in beta cells. Diabetes 57, Suppl. 1, A55.
-
- Balczon, R., Overstreet, K. A., Zinkowski, R. P., Haynes, A. and Appel, M. (1992). The identification, purification, and characterization of a pancreatic beta-cell form of the microtubule adenosine triphosphatase kinesin. Endocrinology 131, 331-336. - PubMed
-
- Barg, S., Huang, P., Eliasson, L., Nelson, D. J., Obermuller, S., Rorsman, P., Thevenod, F. and Renstrom, E. (2001). Priming of insulin granules for exocytosis by granular Cl(-) uptake and acidification. J. Cell Sci. 114, 2145-2154. - PubMed
-
- Barg, S., Eliasson, L., Renstrom, E. and Rorsman, P. (2002). A subset of 50 secretory granules in close contact with L-type Ca(2+) channels accounts for first-phase insulin secretion in mouse beta-cells. Diabetes 51, S74-S82. - PubMed
-
- Berglund, O. (1980). Different dynamics of insulin secretion in the perfused pancreas of mouse and rat. Acta Endocrinol. 93, 54-60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

