Laminin is required for Schwann cell morphogenesis
- PMID: 19295124
- PMCID: PMC2720928
- DOI: 10.1242/jcs.033928
Laminin is required for Schwann cell morphogenesis
Abstract
Development of the peripheral nervous system requires radial axonal sorting by Schwann cells (SCs). To accomplish sorting, SCs must both proliferate and undergo morphogenetic changes such as process extension. Signaling studies reveal pathways that control either proliferation or morphogenesis, and laminin is essential for SC proliferation. However, it is not clear whether laminin is also required for SC morphogenesis. By using a novel time-lapse live-cell-imaging technique, we demonstrated that laminins are required for SCs to form a bipolar shape as well as for process extension. These morphological deficits are accompanied by alterations in signaling pathways. Phosphorylation of Schwannomin at serine 518 and activation of Rho GTPase Cdc42 and Rac1 were all significantly decreased in SCs lacking laminins. Inhibiting Rac1 and/or Cdc42 activities in cultured SCs attenuated laminin-induced myelination, whereas forced activation of Rac1 and/or Cdc42 in vivo improved sorting and hypomyelinating phenotypes in SCs lacking laminins. These findings indicate that laminins play a pivotal role in regulating SC cytoskeletal signaling. Coupled with previous results demonstrating that laminin is critical for SC proliferation, this work identifies laminin signaling as a central regulator coordinating the processes of proliferation and morphogenesis in radial axonal sorting.
Figures
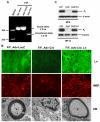

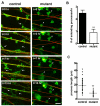

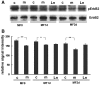

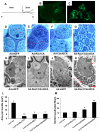
Similar articles
-
Beta1 integrin activates Rac1 in Schwann cells to generate radial lamellae during axonal sorting and myelination.J Cell Biol. 2007 Jun 18;177(6):1063-75. doi: 10.1083/jcb.200610014. J Cell Biol. 2007. PMID: 17576799 Free PMC article.
-
The atypical Guanine-nucleotide exchange factor, dock7, negatively regulates schwann cell differentiation and myelination.J Neurosci. 2011 Aug 31;31(35):12579-92. doi: 10.1523/JNEUROSCI.2738-11.2011. J Neurosci. 2011. PMID: 21880919 Free PMC article.
-
Schwann cell myelination requires integration of laminin activities.J Cell Sci. 2012 Oct 1;125(Pt 19):4609-19. doi: 10.1242/jcs.107995. Epub 2012 Jul 5. J Cell Sci. 2012. PMID: 22767514 Free PMC article.
-
Myelination: all about Rac 'n' roll.J Cell Biol. 2007 Jun 18;177(6):953-5. doi: 10.1083/jcb.200705105. J Cell Biol. 2007. PMID: 17576794 Free PMC article. Review.
-
Regulation of Schwann cell function by the extracellular matrix.Glia. 2008 Nov 1;56(14):1498-1507. doi: 10.1002/glia.20740. Glia. 2008. PMID: 18803319 Review.
Cited by
-
ErbB signaling has a role in radial sorting independent of Schwann cell number.Glia. 2011 Jul;59(7):1047-55. doi: 10.1002/glia.21175. Epub 2011 Apr 12. Glia. 2011. PMID: 21491500 Free PMC article.
-
New insights into signaling during myelination in zebrafish.Curr Top Dev Biol. 2011;97:1-19. doi: 10.1016/B978-0-12-385975-4.00007-3. Curr Top Dev Biol. 2011. PMID: 22074600 Free PMC article. Review.
-
Intrinsic migratory properties of cultured Schwann cells based on single-cell migration assay.PLoS One. 2012;7(12):e51824. doi: 10.1371/journal.pone.0051824. Epub 2012 Dec 14. PLoS One. 2012. PMID: 23251634 Free PMC article.
-
Enzymatically crosslinked gelatin-laminin hydrogels for applications in neuromuscular tissue engineering.Biomater Sci. 2020 Jan 21;8(2):591-606. doi: 10.1039/c9bm01430f. Biomater Sci. 2020. PMID: 31859298 Free PMC article.
-
Human Platelet Lysate Acts Synergistically With Laminin to Improve the Neurotrophic Effect of Human Adipose-Derived Stem Cells on Primary Neurons in vitro.Front Bioeng Biotechnol. 2021 Mar 19;9:658176. doi: 10.3389/fbioe.2021.658176. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 33816456 Free PMC article.
References
-
- Benninger, Y., Thurnherr, T., Pereira, J. A., Krause, S., Wu, X., Chrostek-Grashoff, A., Herzog, D., Nave, K. A., Franklin, R. J., Meijer, D. et al. (2007). Essential and distinct roles for cdc42 and rac1 in the regulation of Schwann cell biology during peripheral nervous system development. J. Cell Biol. 177, 1051-1061. - PMC - PubMed
-
- Cosgaya, J. M., Chan, J. R. and Shooter, E. M. (2002). The neurotrophin receptor p75NTR as a positive modulator of myelination. Science 298, 1245-1248. - PubMed
-
- Feltri, M. L., Graus Porta, D., Previtali, S. C., Nodari, A., Migliavacca, B., Cassetti, A., Littlewood-Evans, A., Reichardt, L. F., Messing, A., Quattrini, A. et al. (2002). Conditional disruption of beta 1 integrin in Schwann cells impedes interactions with axons. J. Cell Biol. 156, 199-209. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

