Three-dimensional electron microscopy reveals new details of membrane systems for Ca2+ signaling in the heart
- PMID: 19295127
- PMCID: PMC2720931
- DOI: 10.1242/jcs.028175
Three-dimensional electron microscopy reveals new details of membrane systems for Ca2+ signaling in the heart
Abstract
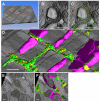
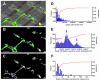
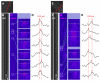
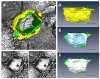
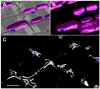
In the current study, the three-dimensional (3D) topologies of dyadic clefts and associated membrane organelles were mapped in mouse ventricular myocardium using electron tomography. The morphological details and the distribution of membrane systems, including transverse tubules (T-tubules), junctional sarcoplasmic reticulum (SR) and vicinal mitochondria, were determined and presumed to be crucial for controlling cardiac Ca(2+) dynamics. The geometric complexity of T-tubules that varied in diameter with frequent branching was clarified. Dyadic clefts were intricately shaped and remarkably small (average 4.39x10(5) nm(3), median 2.81x10(5) nm(3)). Although a dyadic cleft of average size could hold maximum 43 ryanodine receptor (RyR) tetramers, more than one-third of clefts were smaller than the size that is able to package as many as 15 RyR tetramers. The dyadic clefts were also adjacent to one another (average end-to-end distance to the nearest dyadic cleft, 19.9 nm) and were distributed irregularly along T-tubule branches. Electron-dense structures that linked membrane organelles were frequently observed between mitochondrial outer membranes and SR or T-tubules. We, thus, propose that the topology of dyadic clefts and the neighboring cellular micro-architecture are the major determinants of the local control of Ca(2+) in the heart, including the establishment of the quantal nature of SR Ca(2+) releases (e.g. Ca(2+) sparks).
Figures

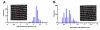

References
-
- Bers, D. M. (2001). Excitation-Contraction Coupling and Cardiac Contractile Force. 2nd edn. Dordrecht, The Netherlands: Kluwer Academic Publishers.
-
- Bers, D. M. and Stiffel, V. M. (1993). Ratio of ryanodine to dihydropyridine receptors in cardiac and skeletal muscle and implications for E-C coupling. Am. J. Physiol. 264, C1587-C1593. - PubMed
-
- Bossen, E. H., Sommer, J. R. and Waugh, R. A. (1978). Comparative stereology of the mouse and finch left ventricle. Tissue Cell 10, 773-784. - PubMed
-
- Brette, F. and Orchard, C. (2007). Resurgence of cardiac t-tubule research. Physiology (Bethesda) 22, 167-173. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

