Cellular plasticity for group I mGluR-mediated epileptogenesis
- PMID: 19295155
- PMCID: PMC2692254
- DOI: 10.1523/JNEUROSCI.5447-08.2009
Cellular plasticity for group I mGluR-mediated epileptogenesis
Abstract
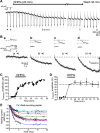
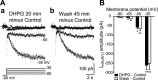
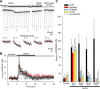
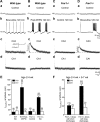
Stimulation of group I metabotropic glutamate receptors (mGluRs) by the agonist (S)-dihydroxyphenylglycine in the hippocampus transforms normal neuronal activity into prolonged epileptiform discharges. The conversion is long lasting in that epileptiform discharges persist after washout of the inducing agonist and serves as a model of epileptogenesis. The group I mGluR model of epileptogenesis took on special significance because epilepsy associated with fragile X syndrome (FXS) may be caused by excessive group I mGluR signaling. At present, the plasticity mechanism underlying the group I mGluR-mediated epileptogenesis is unknown. I(mGluR(V)), a voltage-gated cationic current activated by group I mGluR agonists in CA3 pyramidal cells in the hippocampus, is a possible candidate. I(mGluR(V)) activation is associated with group I mGluR agonist-elicited epileptiform discharges. For I(mGluR(V)) to play a role in epileptogenesis, long-term activation of the current must occur after group I mGluR agonist exposure or synaptic stimulation. We observed that I(mGluR(V)), once induced by group I mGluR agonist stimulation in CA3 pyramidal cells, remained undiminished for hours after agonist washout. In slices prepared from FXS model mice, repeated stimulation of recurrent CA3 pyramidal cell synapses, effective in eliciting mGluR-mediated epileptiform discharges, also induced long-lasting I(mGluR(V)) in CA3 pyramidal cells. Similar to group I mGluR-mediated prolonged epileptiform discharges, persistent I(mGluR(V)) was no longer observed in preparations pretreated with inhibitors of tyrosine kinase, of extracellular signal-regulated kinase 1/2, or of mRNA protein synthesis. The results indicate that I(mGluR(V)) is an intrinsic plasticity mechanism associated with group I mGluR-mediated epileptogenesis.
Figures




References
-
- Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27:370–377. - PubMed
-
- Bianchi R, Young SR, Wong RKS. Group I mGluR activation causes voltage-dependent and -independent Ca2+ rises in hippocampal pyramidal cells. J Neurophysiol. 1999;81:2903–2913. - PubMed
-
- Bianchi R, Chuang SC, Wong RKS. Pharmacology of a slowly inactivating outward current in hippocampal CA3 pyramidal neurons. J Neurophysiol. 2006;96:1116–1123. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
