Th17 cytokines stimulate CCL20 expression in keratinocytes in vitro and in vivo: implications for psoriasis pathogenesis
- PMID: 19295614
- PMCID: PMC2892172
- DOI: 10.1038/jid.2009.65
Th17 cytokines stimulate CCL20 expression in keratinocytes in vitro and in vivo: implications for psoriasis pathogenesis
Abstract
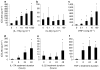
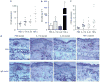
T helper (Th) 17 cells have recently been implicated in psoriasis pathogenesis, but mechanisms of how these cells traffic into inflamed skin are unknown. By immunostaining for interleukin (IL)-17A and IL-22, we show numerous cells present in psoriasis lesions that produce these cytokines. We next found that Th17 cytokines (IL-17A, IL-22, and tumor necrosis factor (TNF)-alpha) markedly increased the expression of CC chemokine ligand (CCL) 20, a CC chemokine receptor (CCR)6 ligand, in human keratinocyte monolayer and raft cultures in a dose- and time-dependent manner. Lastly, we showed in mice that subcutaneous injection with recombinant IL-17A, IL-22, or TNF-alpha led to the upregulation of both CCL20 and CCR6 expression in skin as well as cutaneous T-cell infiltration. Taken together, these data show that Th17 cytokines stimulate CCL20 production in vitro and in vivo, and thus provide a potential explanation of how CCR6-positive Th17 cells maintain their continual presence in psoriasis through a positive chemotactic feedback loop.
Conflict of interest statement
The authors state no conflict of interest.
Figures



References
-
- Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007a;8:942–9. - PubMed
-
- Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007b;8:639–46. - PubMed
-
- Austin LM, Ozawa M, Kikuchi T, Walters IB, Krueger JG. The majority of epidermal T cells in Psoriasis vulgaris lesions can produce type 1 cytokines, interferon-gamma, interleukin-2, and tumor necrosis factor-alpha, defining TC1 (cytotoxic T lymphocyte) and TH1 effector populations: a type 1 differentiation bias is also measured in circulating blood T cells in psoriatic patients. J Invest Dermatol. 1999;113:752–9. - PubMed
-
- Blauvelt A. New concepts in the pathogenesis and treatment of psoriasis: key roles for IL-23, IL-17A and TGF-b1. Expert Rev Dermatol. 2007;2:1–10.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

