Transcriptome analysis of synaptoneurosomes identifies neuroplasticity genes overexpressed in incipient Alzheimer's disease
- PMID: 19295912
- PMCID: PMC2654156
- DOI: 10.1371/journal.pone.0004936
Transcriptome analysis of synaptoneurosomes identifies neuroplasticity genes overexpressed in incipient Alzheimer's disease
Abstract
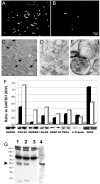
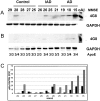
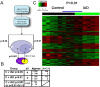
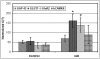
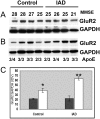
In Alzheimer's disease (AD), early deficits in learning and memory are a consequence of synaptic modification induced by toxic beta-amyloid oligomers (oAbeta). To identify immediate molecular targets downstream of oAbeta binding, we prepared synaptoneurosomes from prefrontal cortex of control and incipient AD (IAD) patients, and isolated mRNAs for comparison of gene expression. This novel approach concentrates synaptic mRNA, thereby increasing the ratio of synaptic to somal mRNA and allowing discrimination of expression changes in synaptically localized genes. In IAD patients, global measures of cognition declined with increasing levels of dimeric Abeta (dAbeta). These patients also showed increased expression of neuroplasticity related genes, many encoding 3'UTR consensus sequences that regulate translation in the synapse. An increase in mRNA encoding the GluR2 subunit of the alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor (AMPAR) was paralleled by elevated expression of the corresponding protein in IAD. These results imply a functional impact on synaptic transmission as GluR2, if inserted, maintains the receptors in a low conductance state. Some overexpressed genes may induce early deficits in cognition and others compensatory mechanisms, providing targets for intervention to moderate the response to dAbeta.
Conflict of interest statement
Figures
Similar articles
-
TrkB activation by 7, 8-dihydroxyflavone increases synapse AMPA subunits and ameliorates spatial memory deficits in a mouse model of Alzheimer's disease.J Neurochem. 2016 Feb;136(3):620-36. doi: 10.1111/jnc.13432. Epub 2015 Dec 29. J Neurochem. 2016. PMID: 26577931
-
Inhibition of calcineurin-mediated endocytosis and alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors prevents amyloid beta oligomer-induced synaptic disruption.J Biol Chem. 2010 Mar 5;285(10):7619-32. doi: 10.1074/jbc.M109.057182. Epub 2009 Dec 23. J Biol Chem. 2010. PMID: 20032460 Free PMC article.
-
Amyloid-beta decreases cell-surface AMPA receptors by increasing intracellular calcium and phosphorylation of GluR2.J Alzheimers Dis. 2010;21(2):655-66. doi: 10.3233/JAD-2010-091654. J Alzheimers Dis. 2010. PMID: 20571220
-
Ca2+-permeable AMPA receptor: A new perspective on amyloid-beta mediated pathophysiology of Alzheimer's disease.Neuropharmacology. 2017 Jan;112(Pt A):221-227. doi: 10.1016/j.neuropharm.2016.08.022. Epub 2016 Aug 22. Neuropharmacology. 2017. PMID: 27561971 Review.
-
Nicotinic receptors, amyloid-beta, and synaptic failure in Alzheimer's disease.J Mol Neurosci. 2010 Jan;40(1-2):221-9. doi: 10.1007/s12031-009-9237-0. Epub 2009 Aug 19. J Mol Neurosci. 2010. PMID: 19690986 Review.
Cited by
-
Network Comparison of Inflammation in Colorectal Cancer and Alzheimer's Disease.Biomed Res Int. 2015;2015:205247. doi: 10.1155/2015/205247. Epub 2015 Jul 26. Biomed Res Int. 2015. PMID: 26273596 Free PMC article.
-
Critical Analysis of Particle Detection Artifacts in Synaptosome Flow Cytometry.eNeuro. 2019 Jun 12;6(3):ENEURO.0009-19.2019. doi: 10.1523/ENEURO.0009-19.2019. Print 2019 May/Jun. eNeuro. 2019. PMID: 31118205 Free PMC article.
-
Inverse correlation between Alzheimer's disease and cancer: implication for a strong impact of regenerative propensity on neurodegeneration?BMC Neurol. 2014 Nov 14;14:211. doi: 10.1186/s12883-014-0211-2. BMC Neurol. 2014. PMID: 25394409 Free PMC article.
-
Gene expression profiling in human neurodegenerative disease.Nat Rev Neurol. 2012 Sep;8(9):518-30. doi: 10.1038/nrneurol.2012.156. Epub 2012 Aug 14. Nat Rev Neurol. 2012. PMID: 22890216 Review.
-
Chemical Stimulation of Rodent and Human Cortical Synaptosomes: Implications in Neurodegeneration.Cells. 2021 May 12;10(5):1174. doi: 10.3390/cells10051174. Cells. 2021. PMID: 34065927 Free PMC article.
References
-
- Selkoe DJ. Alzheimer's Disease Is a Synaptic Failure. Science. 2002;298:789–791. - PubMed
-
- Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, et al. Physical basis of cognitive alterations in Alzheimer's disease: Synapse loss is the major correlate of cognitive impairment. 1991;30:572–580. - PubMed
-
- Masliah E, Mallory M, Alford M, DeTeresa R, Hansen LA, et al. Altered expression of synaptic proteins occurs early during progression of Alzheimer's disease. Neurology. 2001;56:127–129. - PubMed
-
- Cummings BJ, Cotman CW. Image analysis of [beta]-amyloid load in Alzheimer's disease and relation to dementia severity. The Lancet. 1995;346:1524–1528. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

