Taurine supplementation prevents ethanol-induced decrease in serum adiponectin and reduces hepatic steatosis in rats
- PMID: 19296466
- PMCID: PMC2677130
- DOI: 10.1002/hep.22811
Taurine supplementation prevents ethanol-induced decrease in serum adiponectin and reduces hepatic steatosis in rats
Abstract
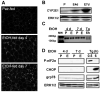
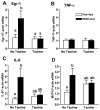
Chronic ethanol feeding decreases expression of adiponectin by adipocytes and circulating adiponectin. Adiponectin treatment during chronic ethanol feeding prevents liver injury in mice. Chronic ethanol feeding also increases oxidative and endoplasmic reticulum (ER) stress in adipose tissue. Here we tested the hypothesis that supplemental taurine, an amino acid that functions as a chemical chaperone/osmolyte and enhances cellular antioxidant activity, would prevent ethanol-induced decreases in adiponectin expression and attenuate liver injury. Serum adiponectin concentrations decreased as early as 4 to 7 days after feeding rats a 36% ethanol diet. This rapid decrease was associated with increased oxidative, but not ER, stress in subcutaneous adipose tissue. Taurine prevented ethanol-induced oxidative stress and increased inflammatory cytokine expression in adipose tissue. Ethanol feeding also rapidly decreased expression of transcription factors regulating adiponectin expression (CCAAT/enhancer binding protein alpha; peroxisome proliferator-activated receptor alpha/gamma) in subcutaneous adipose tissue. Taurine prevented the ethanol-induced decrease in CCAAT/enhancer binding protein alpha and peroxisome proliferator-activated receptor alpha, normalizing adiponectin messenger (m)RNA and serum adiponectin concentrations. In the liver, taurine prevented ethanol-induced oxidative stress and attenuated tumor necrosis factor alpha expression and steatosis, at least in part, by increasing expression of genes involved in fatty acid oxidation.
Conclusion: In subcutaneous adipose tissue, taurine decreased ethanol-induced oxidative stress and cytokine expression, as well as normalized expression of adiponectin mRNA. Taurine prevented ethanol-induced decreases in serum adiponectin; normalized adiponectin was associated with a reduction in hepatic oxidative stress, tumor necrosis factor alpha expression, and steatosis. Taken together, these data demonstrate that taurine has important protective effects against ethanol-induced tissue injury in both adipose and liver tissue.
Figures






References
-
- Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26(3):439–51. - PubMed
-
- Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20(6):1595–9. - PubMed
-
- Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996;271(18):10697–703. - PubMed
-
- Ajuwon KM, Spurlock ME. Adiponectin inhibits LPS-induced NF-kappaB activation and IL-6 production and increases PPARgamma2 expression in adipocytes. Am J Physiol. 2005;288(5):R1220–5. - PubMed
-
- Dey A, Cederbaum AI. Alcohol and oxidative liver injury. Hepatology. 2006;43(2 Suppl 1):S63–74. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
