Characterizing loop dynamics and ligand recognition in human- and avian-type influenza neuraminidases via generalized born molecular dynamics and end-point free energy calculations
- PMID: 19296611
- PMCID: PMC2665887
- DOI: 10.1021/ja8085643
Characterizing loop dynamics and ligand recognition in human- and avian-type influenza neuraminidases via generalized born molecular dynamics and end-point free energy calculations
Abstract
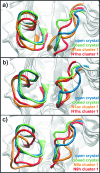
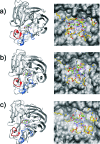
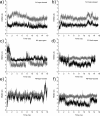
The comparative dynamics and inhibitor binding free energies of group-1 and group-2 pathogenic influenza A subtype neuraminidase (NA) enzymes are of fundamental biological interest and relevant to structure-based drug design studies for antiviral compounds. In this work, we present seven generalized Born molecular dynamics simulations of avian (N1)- and human (N9)-type NAs in order to probe the comparative flexibility of the two subtypes, both with and without the inhibitor oseltamivir bound. The enhanced sampling obtained through the implicit solvent treatment suggests several provocative insights into the dynamics of the two subtypes, including that the group-2 enzymes may exhibit similar motion in the 430-binding site regions but different 150-loop motion. End-point free energy calculations elucidate the contributions to inhibitor binding free energies and suggest that entropic considerations cannot be neglected when comparing across the subtypes. We anticipate the findings presented here will have broad implications for the development of novel antiviral compounds against both seasonal and pandemic influenza strains.
Figures
References
-
- Weekly Epidemiol. Rec. 2003, 78, 49–50(Influenza A (H5N1) in Hong Kong Special Administrative Region of China).
-
- Thompson J. D.; Higgins D. G.; Gibson T. J. Comput. Appl. Biosci. 1994, 10, 19–29. - PubMed
-
- Gubareva L. V.; Kaiser L.; Matrosovich M. N.; Soo-Hoo Y.; Hayden F. G. J. Infect. Dis. 2001, 183, 523–531. - PubMed

