Arf GAP2 is positively regulated by coatomer and cargo
- PMID: 19296914
- PMCID: PMC2692659
- DOI: 10.1016/j.cellsig.2009.03.006
Arf GAP2 is positively regulated by coatomer and cargo
Abstract
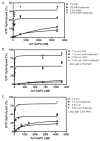
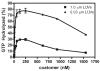
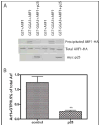
Arf GAP2 is one of four Arf GAPs that function in the Golgi apparatus. We characterized the kinetics of Arf GAP2 and its regulation. Purified Arf GAP2 had little activity compared to purified Arf GAP1. Of the potential regulators we examined, coatomer had the greatest effect, stimulating activity one to two orders of magnitude. The effect was biphasic, with half-maximal activation observed at 50 nM coatomer and activation peaking at approximately 150 nM coatomer. Activation by coatomer was greater for Arf GAP2 than has been reported for Arf GAP1. The effects of phosphoinositides and changes in vesicle curvature on GAP activity were small compared to coatomer; however, both increased coatomer-dependent activity. Peptides from p24 cargo proteins increased Arf GAP2 activity by an additional 2- to 4-fold. The effect of cargo peptide was dependent on coatomer. Overexpressing the cargo protein p25 decreased cellular Arf1*GTP levels. The differential sensitivity of Arf GAP1 and Arf GAP2 to coatomer could coordinate their activities. Based on the common regulatory features of Arf GAP1 and 2, we propose a mechanism for cargo selection in which GTP hydrolysis triggered by cargo binding to the coat protein is coupled to coat polymerization.
Figures






References
-
- Donaldson JG, Lippincott-Schwartz J. Cell. 2000;101:693–696. - PubMed
-
- Bonifacino JS, Glick BS. Cell. 2004;116:153–166. - PubMed
-
- De Matteis MA, Luini A. Nature Rev Mol Cell Biol. 2008;9:273–284. - PubMed
-
- Bonifacino JS, Lippincott-Schwartz J. Nature Rev Mol Cell Biol. 2003;4:409–414. - PubMed
-
- D’Angelo G, Vicinanza M, Di Campli A, De Matteis MA. J Cell Sci. 2008;121:1955–1963. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

