FoxO1 controls insulin-dependent adipose triglyceride lipase (ATGL) expression and lipolysis in adipocytes
- PMID: 19297333
- PMCID: PMC2679428
- DOI: 10.1074/jbc.C800241200
FoxO1 controls insulin-dependent adipose triglyceride lipase (ATGL) expression and lipolysis in adipocytes
Abstract
FoxO1 represents a central regulator of metabolism in several cell types. Although FoxO1 is abundant in adipocytes, its biological functions in these cells remain largely unknown. We show here that the promotor region of the rate-limiting lipolytic enzyme, adipose triglyceride lipase (ATGL), has two FoxO1-binding sites, and co-transfection with wild type and unphosphorylated FoxO1 mutant activates the expression of luciferase driven by the ATGL promotor. In 3T3-L1 adipocytes, insulin controls nucleo-cytoplasmic shuttling of FoxO1 and regulates its interaction with endogenous ATGL promotors. Knockdown of FoxO1 in 3T3-L1 adipocytes decreases the expression of ATGL and attenuates basal and isoproterenol-stimulated lipolysis. Infection of mouse embryonic fibroblasts with FoxO1-encoding lentivirus increases ATGL expression and renders it sensitive to regulation by insulin. Thus, FoxO1 may play an important role in the regulation of lipolysis in adipocytes by controlling the expression of ATGL.
Figures
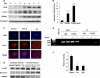
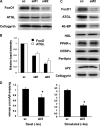
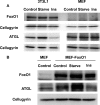
References
-
- Fraze, E., Donner, C. C., Swislocki, A. L., Chiou, Y. A., Chen, Y. D., and Reaven, G. M. (1985) J. Clin. Endocrinol. Metab. 61 807–811 - PubMed
-
- Stumvoll, M., Goldstein, B. J., and van Haeften, T. W. (2008) Lancet 371 2153–2156 - PubMed
-
- Wymann, M. P., and Schneiter, R. (2008) Nat. Rev. Mol. Cell Biol. 9 162–176 - PubMed
-
- Zechner, R., Kienesberger, P. C., Haemmerle, G., Zimmermann, R., and Lass, A. (2009) J. Lipid Res. 50 3–21 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

