Comparison of tocilizumab monotherapy versus methotrexate monotherapy in patients with moderate to severe rheumatoid arthritis: the AMBITION study
- PMID: 19297346
- PMCID: PMC3747519
- DOI: 10.1136/ard.2008.105197
Comparison of tocilizumab monotherapy versus methotrexate monotherapy in patients with moderate to severe rheumatoid arthritis: the AMBITION study
Abstract
Background: The anti-interleukin (IL) 6 receptor antibody tocilizumab inhibits signalling of IL6, a key cytokine in rheumatoid arthritis (RA) pathogenesis.
Objective: To evaluate through the AMBITION study the efficacy and safety of tocilizumab monotherapy versus methotrexate in patients with active RA for whom previous treatment with methotrexate/biological agents had not failed.
Methods: This 24-week, double-blind, double-dummy, parallel-group study, randomised 673 patients to either tocilizumab 8 mg/kg every 4 weeks, or methotrexate, starting at 7.5 mg/week and titrated to 20 mg/week within 8 weeks, or placebo for 8 weeks followed by tocilizumab 8 mg/kg. The primary end point was the proportion of patients achieving American College of Rheumatology (ACR) 20 response at week 24.
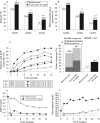
Results: The intention-to-treat analysis demonstrated that tocilizumab was better than methotrexate treatment with a higher ACR20 response (69.9 vs 52.5%; p<0.001), and 28-joint Disease Activity Score (DAS28) <2.6 rate (33.6 vs 12.1%) at week 24. Mean high-sensitivity C-reactive protein was within the normal range from week 12 with tocilizumab, whereas levels remained elevated with methotrexate. The incidence of serious adverse events with tocilizumab was 3.8% versus 2.8% with methotrexate (p = 0.50), and of serious infections, 1.4% versus 0.7%, respectively. There was a higher incidence of reversible grade 3 neutropenia (3.1% vs 0.4%) and increased total cholesterol > or =240 mg/dl (13.2% vs 0.4%), and a lower incidence of alanine aminotransferase elevations >3x-<5x upper limit of normal (1.0% vs 2.5%), respectively.
Conclusion: Tocilizumab monotherapy is better than methotrexate monotherapy, with rapid improvement in RA signs and symptoms, and a favourable benefit-risk, in patients for whom treatment with methotrexate or biological agents has not previously failed.
Trial registration: ClinicalTrials.gov NCT00109408.
Conflict of interest statement
Figures

References
-
- Choy EH, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med 2001;344:907–16 - PubMed
-
- Sack U, Kinne RW, Marx T, Heppt P, Bender S, Emmrich F. Interleukin-6 in synovial fluid is closely associated with chronic synovitis in rheumatoid arthritis. Rheumatol Int 1993;13:45–51 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

