Common genetic control of haemangioblast and cardiac development in zebrafish
- PMID: 19297410
- PMCID: PMC2730399
- DOI: 10.1242/dev.032748
Common genetic control of haemangioblast and cardiac development in zebrafish
Abstract
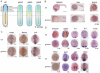
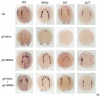
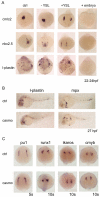
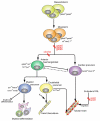
Over the past few years it has become clear that over half of the mammalian heart derives from outside the heart field as originally defined. Such a second heart field, however, has not been described in zebrafish, which could explain its smaller, two-chambered heart. Instead, zebrafish have a population of haemangioblasts, which is absent in mammalian embryos, raising the possibility that these cells represent the evolutionary ancestor of the second heart field. Here, we show for the first time that the genetic programmes of these anterior haemangioblasts and the adjacent heart field are co-regulated, by transcription factors previously associated with heart but not blood or endothelial development. We demonstrate that gata4, gata5 and gata6 are essential for anterior haemangioblast specification, and for subsequent myelopoiesis, acting as early as cloche and upstream of scl. The requirement for gata4, gata5 and gata6 in myeloid, endothelial and cardiac specification is in the mesoderm, but these factors also control, from within the endoderm and the yolk syncytial layer, the migration of the cardiac precursors as they differentiate. This genetic link between the blood/endothelial and cardiac programmes supports the notion that this haemangioblast population in zebrafish is an evolutionary antecedent of the second heart field, and has implications for the differentiation of haemangioblasts and cardiomyocytes from pluripotent cells, and for the origins of stem cells in the adult heart.
Figures



References
-
- Ahn, D. G., Kourakis, M. J., Rohde, L. A., Silver, L. M. and Ho, R. K. (2002). T-box gene tbx5 is essential for formation of the pectoral limb bud. Nature 417, 754-758. - PubMed
-
- Alexander, J., Rothenberg, M., Henry, G. L. and Stainier, D. Y. (1999). casanova plays an early and essential role in endoderm formation in zebrafish. Dev. Biol. 215, 343-357. - PubMed
-
- Baird, J. W., Ryan, K. M., Hayes, I., Hampson, L., Heyworth, C. M., Clark, A., Wootton, M., Ansell, J. D., Menzel, U., Hole, N. et al. (2001). Differentiating embryonal stem cells are a rich source of haemopoietic gene products and suggest erythroid preconditioning of primitive haemopoietic stem cells. J. Biol. Chem. 276, 9189-9198. - PubMed
-
- Belaoussoff, M., Farrington, S. M. and Baron, M. H. (1998). Hematopoietic induction and respecification of A-P identity by visceral endoderm signaling in the mouse embryo. Development 125, 5009-5018. - PubMed
-
- Bielinska, M., Narita, N., Heikinheimo, M., Porter, S. B. and Wilson, D. B. (1996). Erythropoiesis and vasculogenesis in embryoid bodies lacking visceral yolk sac endoderm. Blood 88, 3720-3730. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

