Type I and type II interferons inhibit the translation of murine norovirus proteins
- PMID: 19297466
- PMCID: PMC2681988
- DOI: 10.1128/JVI.00231-09
Type I and type II interferons inhibit the translation of murine norovirus proteins
Abstract
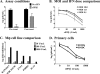
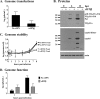
Human noroviruses are responsible for more than 95% of nonbacterial epidemic gastroenteritis worldwide. Both onset and resolution of disease symptoms are rapid, suggesting that components of the innate immune response are critical in norovirus control. While the study of the human noroviruses has been hampered by the lack of small animal and tissue culture systems, our recent discovery of a murine norovirus (MNV) and its in vitro propagation have allowed us to begin addressing norovirus replication strategies and immune responses to norovirus infection. We have previously demonstrated that interferon responses are critical to control MNV-1 infection in vivo and to directly inhibit viral replication in vitro. We now extend these studies to define the molecular basis for interferon-mediated inhibition. Viral replication intermediates were not detected in permissive cells pretreated with type I interferon after either infection or transfection of virion-associated RNA, demonstrating a very early block to virion production that is after virus entry and uncoating. A similar absence of viral replication intermediates was observed in infected primary macrophages and dendritic cells pretreated with type I IFN. This was not due to degradation of incoming genomes in interferon-pretreated cells since similar levels of genomes were present in untreated and pretreated cells through 6 h of infection, and these genomes retained their integrity. Surprisingly, this block to the translation of viral proteins was not dependent on the well-characterized interferon-induced antiviral molecule PKR. Similar results were observed in cells pretreated with type II interferon, except that the inhibition of viral translation was dependent on PKR. Thus, both type I and type II interferon signaling inhibit norovirus translation in permissive myeloid cells, but they display distinct dependence on PKR for this inhibition.
Figures



Similar articles
-
ISG15 functions as an interferon-mediated antiviral effector early in the murine norovirus life cycle.J Virol. 2014 Aug;88(16):9277-86. doi: 10.1128/JVI.01422-14. Epub 2014 Jun 4. J Virol. 2014. PMID: 24899198 Free PMC article.
-
Antiviral activity of Schizonepeta tenuifolia Briquet against noroviruses via induction of antiviral interferons.J Microbiol. 2018 Sep;56(9):683-689. doi: 10.1007/s12275-018-8228-7. Epub 2018 Aug 23. J Microbiol. 2018. PMID: 30141161
-
Critical role for interferon regulatory factor 3 (IRF-3) and IRF-7 in type I interferon-mediated control of murine norovirus replication.J Virol. 2012 Dec;86(24):13515-23. doi: 10.1128/JVI.01824-12. Epub 2012 Oct 3. J Virol. 2012. PMID: 23035219 Free PMC article.
-
The Role of Interferon in Persistent Viral Infection: Insights from Murine Norovirus.Trends Microbiol. 2018 Jun;26(6):510-524. doi: 10.1016/j.tim.2017.10.010. Epub 2017 Nov 17. Trends Microbiol. 2018. PMID: 29157967 Free PMC article. Review.
-
Norovirus gene expression and replication.J Gen Virol. 2014 Feb;95(Pt 2):278-291. doi: 10.1099/vir.0.059634-0. Epub 2013 Nov 16. J Gen Virol. 2014. PMID: 24243731 Review.
Cited by
-
Host-Level Susceptibility and IRF1 Expression Influence the Ability of IFN-γ to Inhibit KSHV Infection in B Lymphocytes.Viruses. 2022 Oct 19;14(10):2295. doi: 10.3390/v14102295. Viruses. 2022. PMID: 36298850 Free PMC article.
-
Norovirus antivirals: Where are we now?Med Res Rev. 2019 May;39(3):860-886. doi: 10.1002/med.21545. Epub 2018 Dec 25. Med Res Rev. 2019. PMID: 30584800 Free PMC article. Review.
-
Bacterial extracellular vesicles control murine norovirus infection through modulation of antiviral immune responses.Front Immunol. 2022 Aug 4;13:909949. doi: 10.3389/fimmu.2022.909949. eCollection 2022. Front Immunol. 2022. PMID: 35990695 Free PMC article.
-
Therapeutics and Immunoprophylaxis Against Noroviruses and Rotaviruses: The Past, Present, and Future.Curr Drug Metab. 2018;19(3):170-191. doi: 10.2174/1389200218666170912161449. Curr Drug Metab. 2018. PMID: 28901254 Free PMC article. Review.
-
Comparative murine norovirus studies reveal a lack of correlation between intestinal virus titers and enteric pathology.Virology. 2011 Dec 20;421(2):202-10. doi: 10.1016/j.virol.2011.09.030. Epub 2011 Oct 22. Virology. 2011. PMID: 22018636 Free PMC article.
References
-
- Burroughs, J. N., and F. Brown. 1978. Presence of a covalently linked protein on calicivirus RNA. J. Gen. Virol. 41443-446. - PubMed
-
- Centers for Disease Control and Prevention. 2005. Infectious disease and dermatologic conditions in evacuees and rescue workers after Hurricane Katrina-multiple states, August-September, 2005. MMWR Morb. Mortal. Wkly. Rep. 54961-964. - PubMed
-
- Centers for Disease Control and Prevention. 2005. Norovirus outbreak among evacuees from hurricane Katrina-Houston, Texas, September 2005. MMWR Morb. Mortal. Wkly. Rep. 541016-1018. - PubMed
-
- Centers for Disease Control and Prevention. 2002. Outbreak of acute gastroenteritis associated with Norwalk-like viruses among British military personnel-Afghanistan, May 2002. MMWR Morb. Mortal. Wkly. Rep. 51477-479. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

