Early and strong immune responses are associated with control of viral replication and recovery in lassa virus-infected cynomolgus monkeys
- PMID: 19297492
- PMCID: PMC2681932
- DOI: 10.1128/JVI.01948-08
Early and strong immune responses are associated with control of viral replication and recovery in lassa virus-infected cynomolgus monkeys
Abstract
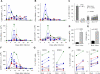
Lassa virus causes a hemorrhagic fever endemic in West Africa. The pathogenesis and the immune responses associated with the disease are poorly understood, and no vaccine is available. We followed virological, pathological, and immunological markers associated with fatal and nonfatal Lassa virus infection of cynomolgus monkeys. The clinical picture was characterized by fever, weight loss, depression, and acute respiratory syndrome. Transient thrombocytopenia and lymphopenia, lymphadenopathy, splenomegaly, infiltration of mononuclear cells, and alterations of the liver, lungs, and endothelia were observed. Survivors exhibited fewer lesions and a lower viral load than nonsurvivors. Although all animals developed strong humoral responses, antibodies appeared more rapidly in survivors and were directed against GP(1), GP(2), and NP. Type I interferons were detected early after infection in survivors but only during the terminal stages in fatalities. The mRNAs for CXCL10 (IP-10) and CXCL11 (I-TAC) were abundant in peripheral blood mononuclear cells and lymph nodes from infected animals, but plasma interleukin-6 was detected only in fatalities. In survivors, high activated-monocyte counts were followed by a rise in the total number of circulating monocytes. Activated T lymphocytes circulated in survivors, whereas T-cell activation was low and delayed in fatalities. In vitro stimulation with inactivated Lassa virus induced activation of T lymphocytes from all infected monkeys, but only lymphocytes from survivors proliferated. Thus, early and strong immune responses and control of viral replication were associated with recovery, whereas fatal infection was characterized by major alterations of the blood formula and, in organs, weak immune responses and uncontrolled viral replication.
Figures


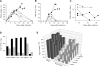

References
-
- Baize, S., J. Kaplon, C. Faure, D. Pannetier, M. C. Georges-Courbot, and V. Deubel. 2004. Lassa virus infection of dendritic cells and macrophages is productive but fails to activate cells. J. Immunol. 1722861-2869. - PubMed
-
- Baize, S., D. Pannetier, C. Faure, P. Marianneau, I. Marendat, M. C. Georges-Courbot, and V. Deubel. 2006. Role of interferons in the control of Lassa virus replication in human dendritic cells and macrophages. Microbes Infect. 81194-1202. - PubMed
-
- Borish, L., R. Rosenbaum, L. Albury, and S. Clark. 1989. Activation of neutrophils by recombinant interleukin 6. Cell. Immunol. 121280-289. - PubMed
-
- Bruns, M., A. Gessner, H. Lother, and F. Lehmann-Grube. 1988. Host cell-dependent homologous interference in lymphocytic choriomeningitis virus infection. Virology 166133-139. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

