Evidence that productive human immunodeficiency virus type 1 assembly can occur in an intracellular compartment
- PMID: 19297499
- PMCID: PMC2681934
- DOI: 10.1128/JVI.00109-09
Evidence that productive human immunodeficiency virus type 1 assembly can occur in an intracellular compartment
Abstract
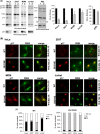
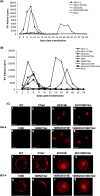
Human immunodeficiency virus type 1 (HIV-1) assembly occurs predominantly at the plasma membrane of infected cells. The targeting of assembly to intracellular compartments such as multivesicular bodies (MVBs) generally leads to a significant reduction in virus release efficiency, suggesting that MVBs are a nonproductive site for HIV-1 assembly. In the current study, we make use of an HIV-1 Gag-matrix mutant, 29/31KE, that is MVB targeted. We previously showed that this mutant is severely defective for virus particle production in HeLa cells but more modestly affected in primary macrophages. To more broadly examine the consequences of MVB targeting for virus production, we investigated 29/31KE particle production in a range of cell types. Surprisingly, this mutant supported highly efficient assembly and release in T cells despite its striking MVB Gag localization. Manipulation of cellular endocytic pathways revealed that unlike Vpu-defective HIV-1, which demonstrated intracellular Gag localization as a result of Gag endocytosis from the plasma membrane, 29/31KE mutant Gag was targeted directly to an MVB compartment. The 29/31KE mutant was unable to support multiple-round replication; however, this defect could be reversed by truncating the cytoplasmic tail of the transmembrane envelope glycoprotein gp41 and by the acquisition of a 16EK change in matrix. The 16EK/29/31KE matrix mutant replicated efficiently in the MT-4 T-cell line despite maintaining an MVB-targeting phenotype. These results indicate that MVB-targeted Gag can be efficiently released from T cells and primary macrophages, suggesting that under some circumstances, late endosomal compartments can serve as productive sites for HIV-1 assembly in these physiologically relevant cell types.
Figures






References
-
- Adamson, C. S., and E. O. Freed. 2007. Human immunodeficiency virus type 1 assembly, release, and maturation. Adv. Pharmacol. 55347-387. - PubMed
-
- Batonick, M., M. Favre, M. Boge, P. Spearman, S. Honing, and M. Thali. 2005. Interaction of HIV-1 Gag with the clathrin-associated adaptor AP-2. Virology 342190-200. - PubMed
-
- Bieniasz, P. D. 2006. Late budding domains and host proteins in enveloped virus release. Virology 34455-63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

