Down-regulation of the met receptor tyrosine kinase by presenilin-dependent regulated intramembrane proteolysis
- PMID: 19297528
- PMCID: PMC2675628
- DOI: 10.1091/mbc.e08-09-0969
Down-regulation of the met receptor tyrosine kinase by presenilin-dependent regulated intramembrane proteolysis
Abstract
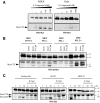
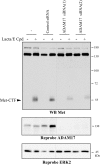
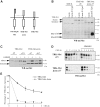
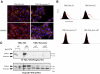
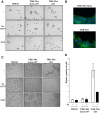
Hepatocyte growth factor/scatter factor (HGF/SF) acts through the membrane-anchored Met receptor tyrosine kinase to induce invasive growth. Deregulation of this signaling is associated with tumorigenesis and involves, in most cases, overexpression of the receptor. We demonstrate that Met is processed in epithelial cells by presenilin-dependent regulated intramembrane proteolysis (PS-RIP) independently of ligand stimulation. The proteolytic process involves sequential cleavage by metalloproteases and the gamma-secretase complex, leading to generation of labile fragments. In normal epithelial cells, although expression of cleavable Met by PS-RIP is down-regulated, uncleavable Met displayed membrane accumulation and induced ligand-independent motility and morphogenesis. Inversely, in transformed cells, the Met inhibitory antibody DN30 is able to promote Met PS-RIP, resulting in down-regulation of the receptor and inhibition of the Met-dependent invasive growth. This demonstrates the original involvement of a proteolytic process in degradation of the Met receptor implicated in negative regulation of invasive growth.
Figures


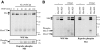

References
-
- Birchmeier C., Birchmeier W., Gherardi E., Vande Woude G. F. Met, metastasis, motility and more. Nat. Rev. Mol. Cell Biol. 2003;4:915–925. - PubMed
-
- Bladt F., Riethmacher D., Isenmann S., Aguzzi A., Birchmeier C. Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature. 1995;376:768–771. - PubMed
-
- Brou C., Logeat F., Gupta N., Bessia C., LeBail O., Doedens J. R., Cumano A., Roux P., Black R. A., Israel A. A novel proteolytic cleavage involved in Notch signaling: the role of the disintegrin-metalloprotease TACE. Mol. Cell. 2000;5:207–216. - PubMed
-
- Comoglio P. M., Giordano S., Trusolino L. Drug development of MET inhibitors: targeting oncogene addiction and expedience. Nat. Rev. Drug Discov. 2008;7:504–516. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

