Wnt/beta-catenin signaling promotes renal interstitial fibrosis
- PMID: 19297557
- PMCID: PMC2663839
- DOI: 10.1681/ASN.2008060566
Wnt/beta-catenin signaling promotes renal interstitial fibrosis
Abstract
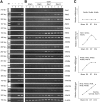
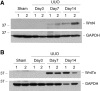
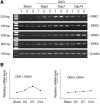
Wnts compose a family of signaling proteins that play an essential role in kidney development, but their expression in adult kidney is thought to be silenced. Here, we analyzed the expression and regulation of Wnts and their receptors and antagonists in normal and fibrotic kidneys after obstructive injury. In the normal mouse kidney, the vast majority of 19 different Wnts and 10 frizzled receptor genes was expressed at various levels. After unilateral ureteral obstruction, all members of the Wnt family except Wnt5b, Wnt8b, and Wnt9b were upregulated in the fibrotic kidney with distinct dynamics. In addition, the expression of most Fzd receptors and Wnt antagonists was also induced. Obstructive injury led to a dramatic accumulation of beta-catenin in the cytoplasm and nuclei of renal tubular epithelial cells, indicating activation of the canonical pathway of Wnt signaling. Numerous Wnt/beta-catenin target genes (c-Myc, Twist, lymphoid enhancer-binding factor 1, and fibronectin) were induced, and their expression was closely correlated with renal beta-catenin abundance. Delivery of the Wnt antagonist Dickkopf-1 gene significantly reduced renal beta-catenin accumulation and inhibited the expression of Wnt/beta-catenin target genes. Furthermore, gene therapy with Dickkopf-1 inhibited myofibroblast activation; suppressed expression of fibroblast-specific protein 1, type I collagen, and fibronectin; and reduced total collagen content in the model of obstructive nephropathy. In summary, these results establish a role for Wnt/beta-catenin signaling in the pathogenesis of renal fibrosis and identify this pathway as a potential therapeutic target.
Figures








Similar articles
-
Wnt-dependent beta-catenin signaling is activated after unilateral ureteral obstruction, and recombinant secreted frizzled-related protein 4 alters the progression of renal fibrosis.J Am Soc Nephrol. 2005 Aug;16(8):2373-84. doi: 10.1681/ASN.2004110949. Epub 2005 Jun 8. J Am Soc Nephrol. 2005. PMID: 15944336
-
Loss of Klotho contributes to kidney injury by derepression of Wnt/β-catenin signaling.J Am Soc Nephrol. 2013 Apr;24(5):771-85. doi: 10.1681/ASN.2012080865. Epub 2013 Apr 4. J Am Soc Nephrol. 2013. PMID: 23559584 Free PMC article.
-
Disruption of the Dapper3 gene aggravates ureteral obstruction-mediated renal fibrosis by amplifying Wnt/β-catenin signaling.J Biol Chem. 2013 May 24;288(21):15006-14. doi: 10.1074/jbc.M113.458448. Epub 2013 Apr 11. J Biol Chem. 2013. PMID: 23580654 Free PMC article.
-
Targeting the Wnt/β-Catenin Signaling Pathway as a Potential Therapeutic Strategy in Renal Tubulointerstitial Fibrosis.Front Pharmacol. 2021 Aug 16;12:719880. doi: 10.3389/fphar.2021.719880. eCollection 2021. Front Pharmacol. 2021. PMID: 34483931 Free PMC article. Review.
-
Wnt/beta-catenin signaling: a novel target for therapeutic intervention of fibrotic kidney disease.Arch Pharm Res. 2009 Dec;32(12):1653-62. doi: 10.1007/s12272-009-2200-3. Epub 2010 Feb 17. Arch Pharm Res. 2009. PMID: 20162391 Review.
Cited by
-
Selective Wnt/β-Catenin Pathway Activation Concomitant With Sustained Overexpression of miR-21 is Responsible for Aristolochic Acid-Induced AKI-to-CKD Transition.Front Pharmacol. 2021 May 28;12:667282. doi: 10.3389/fphar.2021.667282. eCollection 2021. Front Pharmacol. 2021. PMID: 34122087 Free PMC article.
-
Activation of Wnt11 by transforming growth factor-β drives mesenchymal gene expression through non-canonical Wnt protein signaling in renal epithelial cells.J Biol Chem. 2012 Jun 15;287(25):21290-302. doi: 10.1074/jbc.M112.357202. Epub 2012 May 3. J Biol Chem. 2012. PMID: 22556418 Free PMC article.
-
Primary cilia modulate balance of canonical and non-canonical Wnt signaling responses in the injured kidney.Fibrogenesis Tissue Repair. 2015 Apr 16;8:6. doi: 10.1186/s13069-015-0024-y. eCollection 2015. Fibrogenesis Tissue Repair. 2015. PMID: 25901180 Free PMC article.
-
Blood DNA Methylation Predicts Diabetic Kidney Disease Progression in High Fat Diet-Fed Mice.Nutrients. 2022 Feb 13;14(4):785. doi: 10.3390/nu14040785. Nutrients. 2022. PMID: 35215435 Free PMC article.
-
Combination therapy with paricalcitol and trandolapril reduces renal fibrosis in obstructive nephropathy.Kidney Int. 2009 Dec;76(12):1248-57. doi: 10.1038/ki.2009.346. Epub 2009 Sep 16. Kidney Int. 2009. PMID: 19759524 Free PMC article.
References
-
- Clevers H: Wnt/beta-catenin signaling in development and disease. Cell 127: 469–480, 2006 - PubMed
-
- Pinto D, Clevers H: Wnt control of stem cells and differentiation in the intestinal epithelium. Exp Cell Res 306: 357–363, 2005 - PubMed
-
- Thompson MD, Monga SP: WNT/beta-catenin signaling in liver health and disease. Hepatology 45: 1298–1305, 2007 - PubMed
-
- Fodde R, Brabletz T: Wnt/beta-catenin signaling in cancer stemness and malignant behavior. Curr Opin Cell Biol 19: 150–158, 2007 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

