Malaria primes the innate immune response due to interferon-gamma induced enhancement of toll-like receptor expression and function
- PMID: 19297619
- PMCID: PMC2657593
- DOI: 10.1073/pnas.0809742106
Malaria primes the innate immune response due to interferon-gamma induced enhancement of toll-like receptor expression and function
Abstract
Malaria-induced sepsis is associated with an intense proinflammatory cytokinemia for which the underlying mechanisms are poorly understood. It has been demonstrated that experimental infection of humans with Plasmodium falciparum primes Toll-like receptor (TLR)-mediated proinflammatory responses. Nevertheless, the relevance of this phenomenon during natural infection and, more importantly, the mechanisms by which malaria mediates TLR hyperresponsiveness are unclear. Here we show that TLR responses are boosted in febrile patients during natural infection with P. falciparum. Microarray analyses demonstrated that an extraordinary percentage of the up-regulated genes, including genes involving TLR signaling, had sites for IFN-inducible transcription factors. To further define the mechanism involved in malaria-mediated "priming," we infected mice with Plasmodium chabaudi. The human data were remarkably predictive of what we observed in the rodent malaria model. Malaria-induced priming of TLR responses correlated with increased expression of TLR mRNA in a TLR9-, MyD88-, and IFNgamma-dependent manner. Acutely infected WT mice were highly susceptible to LPS-induced lethality while TLR9(-/-), IL12(-/-) and to a greater extent, IFNgamma(-/-) mice were protected. Our data provide unprecedented evidence that TLR9 and MyD88 are essential to initiate IL12 and IFNgamma responses and favor host hyperresponsiveness to TLR agonists resulting in overproduction of proinflammatory cytokines and the sepsis-like symptoms of acute malaria.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



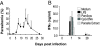
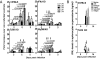
References
-
- Schofield L, Grau GE. Immunological processes in malaria pathogenesis. Nat Rev Immunol. 2005;5:722. - PubMed
-
- Kwiatkowski D, et al. TNF concentration in fatal cerebral, non-fatal cerebral, and uncomplicated Plasmodium falciparum malaria. Lancet. 1990;336:1201. - PubMed
-
- Othoro C, et al. A low interleukin-10 tumor necrosis factor-alpha ratio is associated with malaria anemia in children residing in a holoendemic malaria region in western Kenya. J Infect Dis. 1999;179:279. - PubMed
-
- Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

